Question #04c74
1 Answer
Yes, DNA is acidic in nature, due to the presence of a highly acidic proton on the phosphate in phosphodiester bond between two sugars.
Explanation:
DNA even though being made by four bases namely, Adenine, Guanidine, cytosine and thymine, is still active due to highly acidic proton present on the phosphate in phosphodiester bond between the two nucleotides.
See the image below, it shows the structure of a nucleotide, where three major groups are present
1. Nitrogenous base
2. Pentose sugar
3. Phosphate group
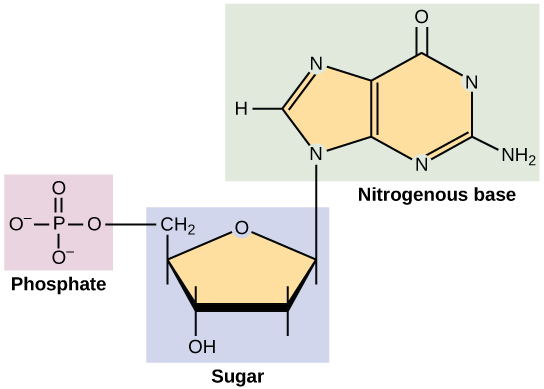 opentextbc.ca/biology/wp-content/uploads/sites/96/2015/08/Figure_02_03_102.jpg
opentextbc.ca/biology/wp-content/uploads/sites/96/2015/08/Figure_02_03_102.jpg
During joining of nucleotides to form DNA fragments you can see formation of phosphodiester bond between the 5- carbon phosphate group and 3 -carbon of adjacent pentose sugar, shown below.

Phosphate group is the one which has the tendency to act as acid. compare the structure of phoshate group in phosphodiester bond (marked with an arrow)with phosphoric acid. As the two hydrogen are replaced by two sugars the remaining hydrogen become quite acidic and most of the time the
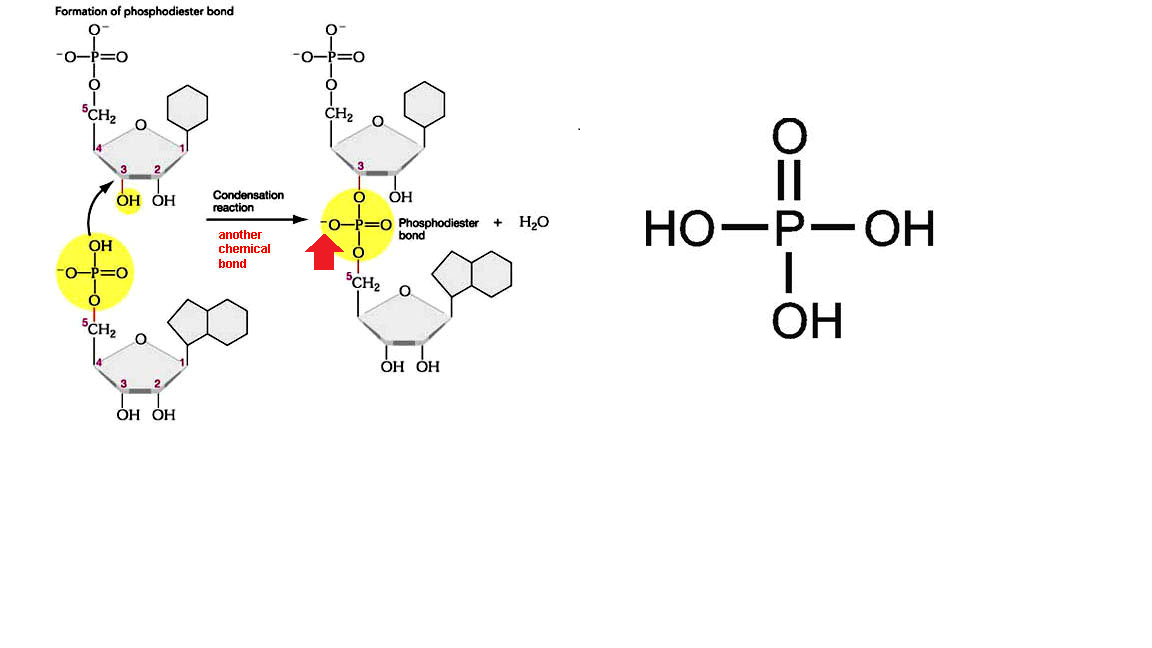 adapted
adapted
*source:images.duckduckgo.com/iu/?u=https%3A%2F%2Fstatic.fishersci.com%2Fimages%2FF158078~wljpg&f=1 and biochemistry3rst.files.wordpress.com/2014/04/phosphodiester-linkagejpg*

