What kinds of molecules are polar?
1 Answer
Molecules with dipole moments that do not cancel out.
Explanation:
Polar molecules are covalent bonded molecules that have a region of positive charge and a region of negative charge. This happens when the electrons are shared unequally, and results in the molecule having a dipole moment.
A polar molecule would be two atoms with high electronegativity differences, and them being covalently bonded together.
A classic example is water.
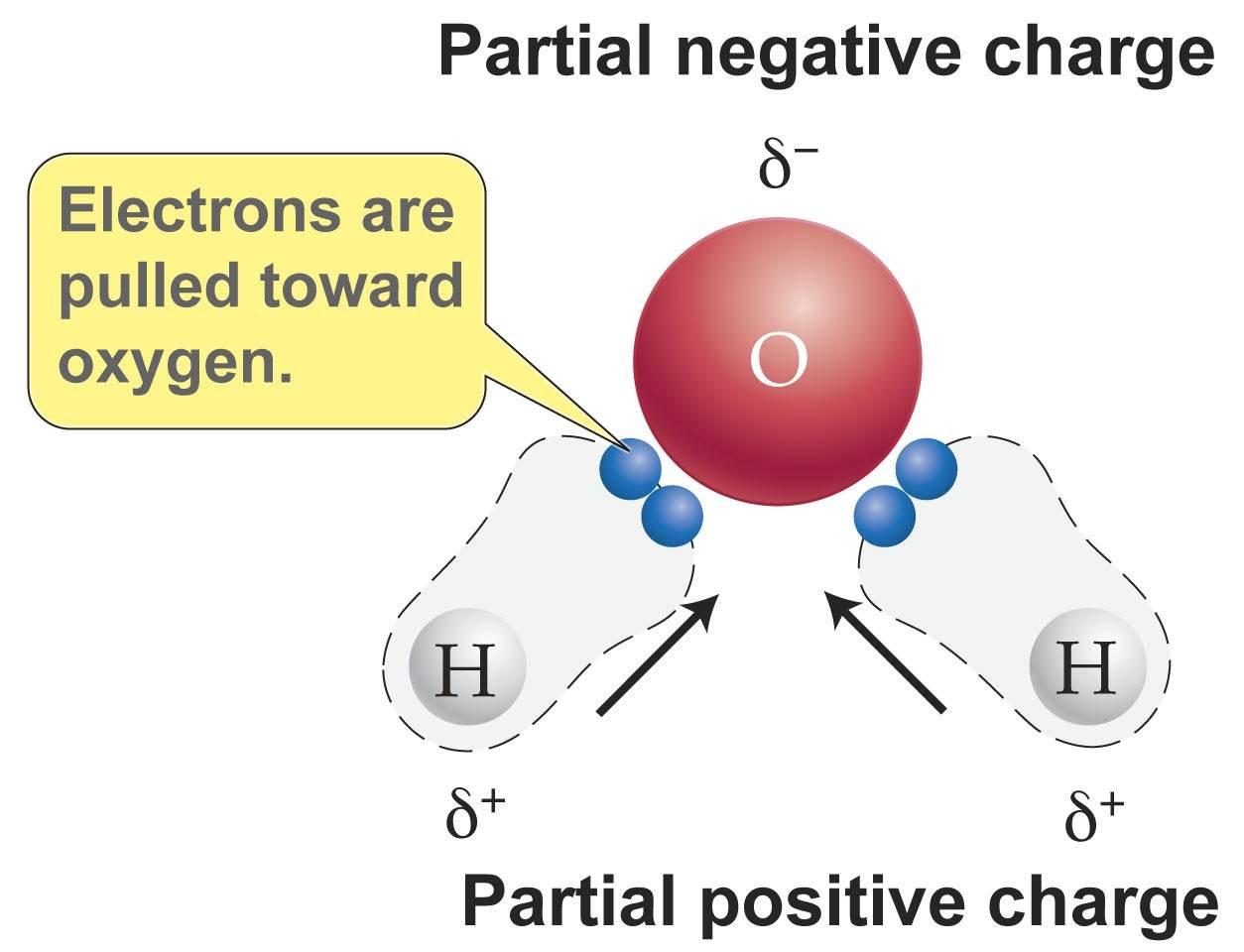
The water molecule is a bent shape, so it is asymmetrical with the dipole moment. The oxygen atom is a lot more electronegative than the hydrogen atom, so electrons from the hydrogen atom get pulled towards the oxygen atom. This results in the hydrogen atoms to be positively-charged region on one end, while the lone unpaired electrons of oxygen form a negatively-charged region on the other hand.
I hope this helps!


