A 350-mL solution contains 12.6 g #NaCl#. What is the molarity of the solution?
1 Answer
Jun 20, 2016
The molarity of the solution is 0.617 M.
Explanation:
Let's use this equation:
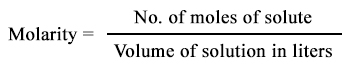
Okay, so we have a bit of work to do! The volume is given in terms of mL and we have to convert that to L by using the following conversion factor:
Now we have to express NaCl in terms of moles. We can do this by using the molar mass of NaCl (58.44 g/mol) as a conversion factor.
Now we can divide the moles by the volume to obtain the molarity like this:
M =0.617

