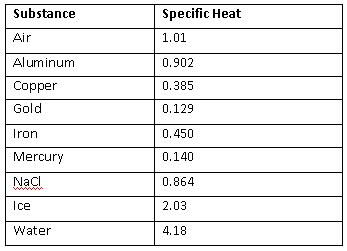
A 36.07 g sample of a substance is initially at 27.8°C. After absorbing 2639 J of heat, the temperature of the substance is 109.0°C. What is the specific heat of the substance?
1 Answer
Jul 25, 2016
I found:
Explanation:
I would use the relationship between heat
so:
rearranging:
Aluminum perhaps?