A chemist dissolved crystals of an unknown substance into water at room temperature. He found that 33 g of the substance can be dissolved into 100 mL of water. What property of the unknown substance was the chemist most likely investigating?
2 Answers
He might have been investigating its solubility.
Explanation:
The solubility of a substance is a measure of how much solute can dissolve in a certain solvent at a given temperature, and is usually expressed in units of grams of solute per
When the chemist found that
The solubilities of substances are specific to each individual solute. That is, in general, no two substances have exactly the same solubility in water at any given temperature. Thus, measuring the water solubility of a substance can also help to identify that substance.
Its solubility in water at room temperature.
Explanation:
The idea here is that the chemist is looking to determine the solubility of the salt in water at room temperature, i.e. the maximum mass of this unknown salt that can be dissolved per
A saturated solution is simply a solution in which the rate at which the solid dissociates to produce solvated ions is equal to the rate at which the solvated ions combine to produce the solid.
#"solid " rightleftharpoons " dissociated ions"#
This implies that the solid can no longer dissolve into the solution.
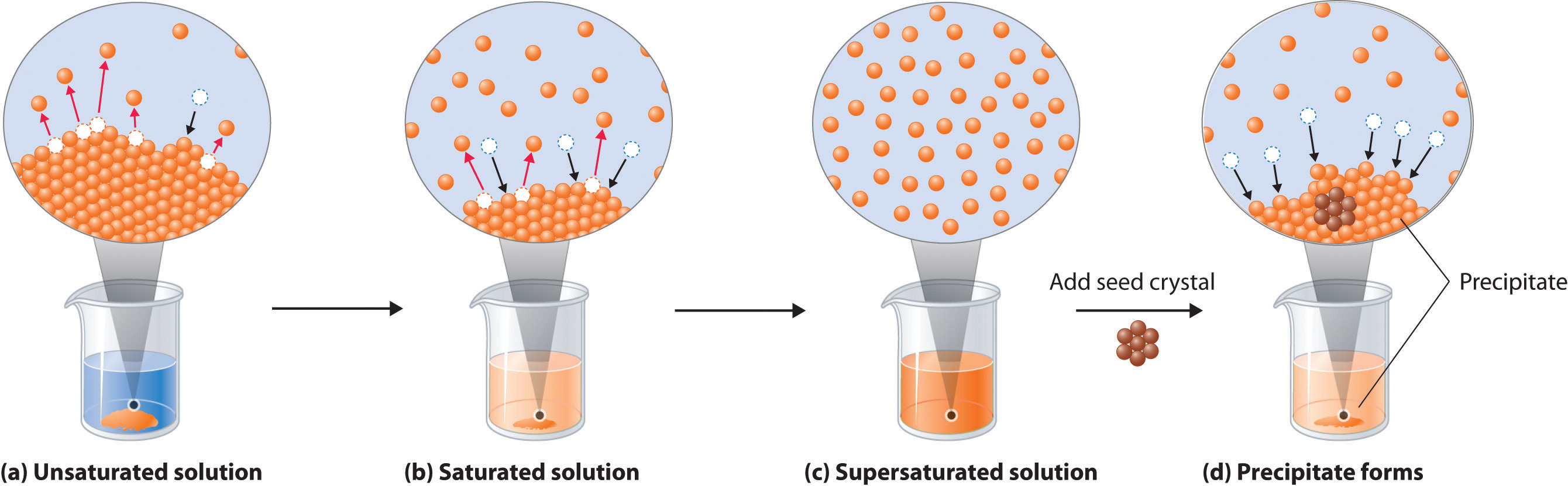
By comparison, an unsaturated solution is a solution in which the rate at which the solid dissociates is higher than the rate at which the ions combine to form the solid.
In this case, the equilibrium lies to the right, so you can represent it as
#"solid " -> " dissociated ions"#
Finally, a supersaturated solution is a solution in which the rate at which the solid dissociates is lower than the rate at which the ions combine to form the solid.
In this case, the equilibrium lies to the left, so you can represent it as
#"dissociated ions " -> " solid"#
In your case, you know that the chemist determined that only
This tells you that at this particular temperature, the solution can only hold
So the equilibrium between the dissociated solid and the solvated ions is reached when you have
#color(darkgreen)(ul(color(black)("solubility = 33 g / 100 mL H"_2"O" )))-># at room temperature
So, for example, if you add
This solution will be unsaturated because it can still hold more dissociated salt, the difference between
If you add
#"53 g " - " 33 g = 20 g"#
of undissociated salt, i.e. solid salt. This solution will be supersaturated at room temperature, i.e. it will hold more solute than it can dissolve.

