A compound with molecular formula C12H24 exhibits a 1H NMR spectrum with only one signal and a 13C NMR spectrum with two signals. What structure could be drawn for this compound?
1 Answer
Jan 22, 2018
I can suggest one candidate....
Explanation:
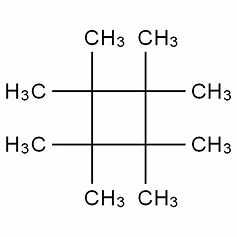
A formula of
And the

