A gas tank holds 3625L of butane at 850mmHg. What is the volume of the butane at 25°C?
The answer is 4054L, by the way
The answer is 4054L, by the way
1 Answer
Have a piece of your teacher; they have no business quoting a pressure with these units of measurement. The question is incomplete in any event.
Explanation:
Mercury has all but disappeared form the modern inorganic laboratory due to safety concerns. In the old days we used a mercury manometer to measure pressure. ONE atmosphere of pressure is known to support a column of mercury that is
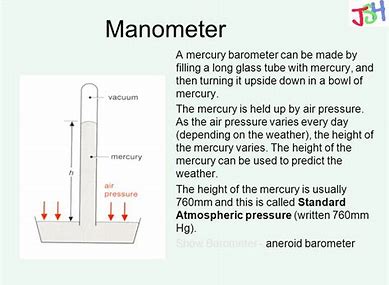 slideplayer.com
slideplayer.com
If you tried to raise the column HIGHER than this, i.e. by putting GREATER than one atmosphere at ONE inlet of the manometer, I have no doubt that you would spray mercury all over the lab, where it would inhabit every nook, and every cranny, and give you a long and arduous clean up job that contract cleaners would not touch.
Just to humour the creature who set the question (who has undoubtedly NEVER used a manometer in his life)....we convert to kosher units...i.e.

