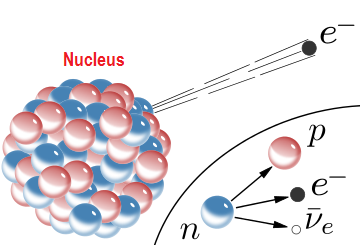
A neutron breaks into a proton and an electron. This decay of neutron is accompanied by the release of energy.Assuming that 50% of the energy is produced in the form of electromagnetic radiation, what will be the frequency of radiation produced.?
1 Answer
1st part
A free neutron decays forming particles such as proton
Here the mass difference occurring for this transformation goes to the formation of electromagnetic radiation.
Initial mass.
the mass of neutron
Final mass
the mass of proton
the mass of electron
So mass difference (neglecting mass of antineutrino
So total energy produced
As per problem
So radiated energy
So frequency of radiation
where
2nd part
Given 1st ionization energy of Al
So this energy is needed to remove first electron from the outermost shell of an atom of Al in its lowest energy state.
The energy of radiation
The energy of the ejected electron


