A piece of copper metal is dipped into an aqueous solution of #AgNO_3#. What is a net ionic equation that describes the reaction that occurs?
1 Answer
Explanation:
This is a single replacement reaction , in which the metals switch places. This can only happen if the metal in the reactants is more reactive than the metal in the products. In order to determine this, we look at an activity series of metals , which is a list of metals in descending order of reactivity. A metal can only replace a metal below it in the activity series.
The diagram below is an activity series of metals. We need to compare where copper is placed relative to silver . In the activity series, it shows that copper is above silver, so this reaction will take place.
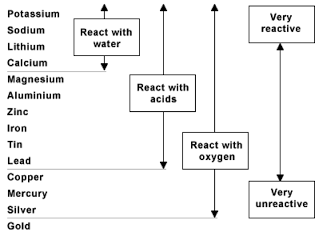
The molecular equation is:
The ionic equation includes all of the ions in the reactants and products.
The net ionic equation includes only the ions that reacted.
Since the nitrate ion occurred on both sides of the equation, it did not participate in the reaction, so we need to cancel it out on both sides. The nitrate ion is called a spectator ion .
The net ionic equation is:

