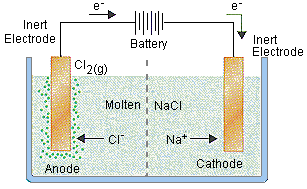
A) predict the products and write the balanced equation including states of matter b) state the type of equation? SODIUM CHLORIDE
HELP is this decomposition or no reaction and why PLEASE EXPLAIN!
HELP is this decomposition or no reaction and why PLEASE EXPLAIN!
1 Answer
Since there is only one reactant, so it appears as if the decomposition of sodium chloride is the reaction.
At first, the sodium metal is in its molten state.
Explanation:
It does seem to indicate that the only reactant is sodium chloride, which would also seem to indicate a decomposition reaction.
In order for the decomposition of sodium chloride to sodium and chlorine gas to occur, it must be in its molten state (i.e. liquid). It also requires an electrical current connected to two inert electrodes, one of which is the anode, which will collect chloride ions