A solution of sodium chloride in water has a vapor pressure of 19.6 torr at 25 degrees Celsius. What is the mole fraction of NaCl solute particles in this solution?
1 Answer
Explanation:
We're asked to find the mole fraction of a solute in solution, given the solution's vapor pressure at
To do this, we can use Raoult's law:
where
-
Psoln is the vapor pressure of the solution -
PoH2O is the vapor pressure of the pure solvent (water) at a given temperature -
χH2O is the mole fraction of water, which we can use to calculate the mole fraction ofNaCl since that is the only other component of the solution
We need to know the vapor pressure of pure water at
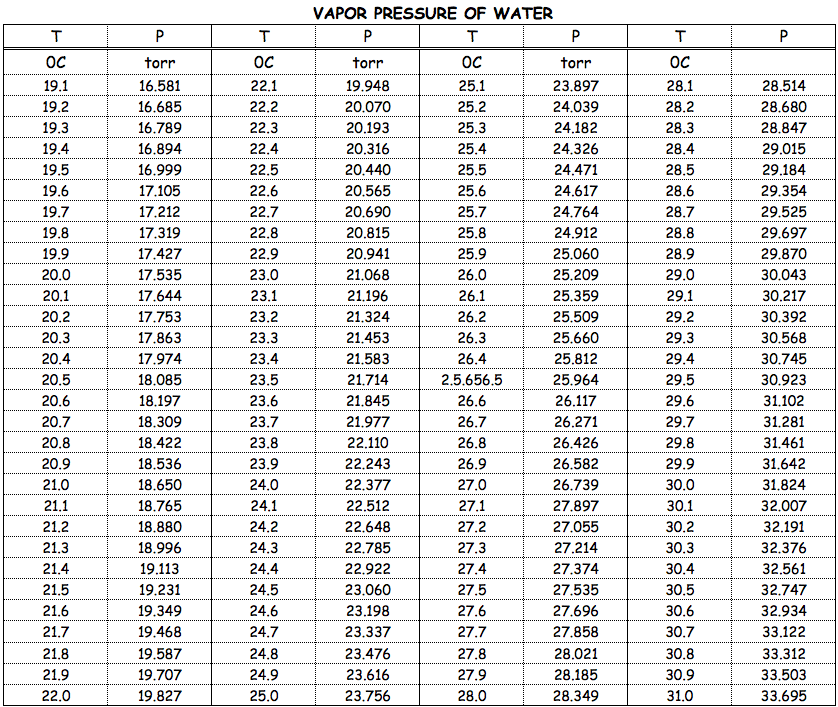 http://faculty.sdmiramar.edu
http://faculty.sdmiramar.edu
We see that the vapor pressure of water at
Our variables:
Plugging in known values:
Which means the mole fraction of
