A volume of hydrogen gas is collected over water in an investigation. If the atmospheric pressure is 102.1 kPa and the pressure of the hydrogen gas is 100.1 kPa, what temperature is the water?
The answer is [ans: 17.5 °C] but I don't know exactly how to get to that answer
The answer is [ans: 17.5 °C] but I don't know exactly how to get to that answer
1 Answer
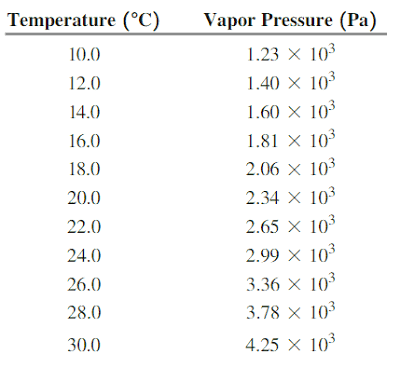
The above table presents different values of pressures of saturated water vapour at different temperatures.
In our problem hydrogen gas has been collected over water. So total pressure (
So
By the problem
So
Now from table we get
At
And
At
This shows that aqueous tension falls by 0.25kPa when temperature falls by
So fall of
So the temperature of the collected gas should be

