An atom contains 11 protons and 11 neutrons. What is the nuclide symbolism for the nucleus?
2 Answers
Jan 18, 2018
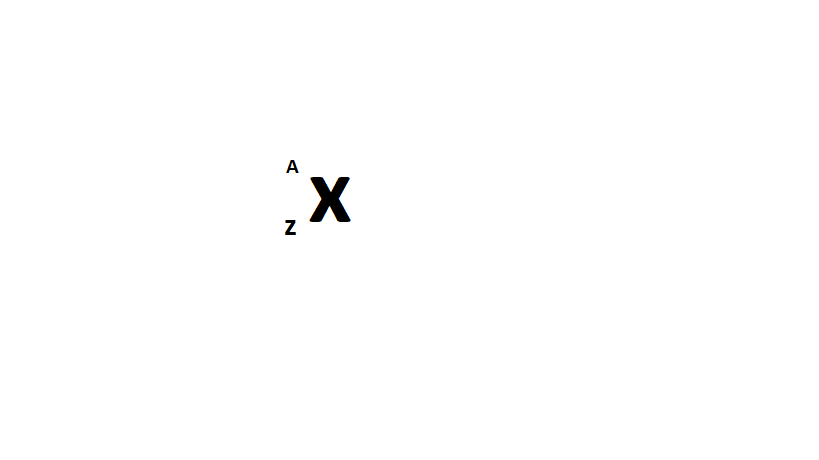
Explanation:
Nuclide symbol is expressed as above, (where,z is the atomic number and A is the mass number)
now, z=total number of protons=11 and a= total number of protons + total number of neutrons=(11+11) or 22
Jan 18, 2018
Explanation:
The number of protons identifies the element. The number of protons is the atomic number, Z. The element with 11 protons is sodium Na. The sum of the protons and neutrons is the mass number, A, and is equal to 22.
The nuclide symbol contains the mass number, A, as a superscript to the left of the element's symbol, and the atomic number, Z, as a subscript to the left of the element's symbol, X:
The nuclide symbol for the given isotope of sodium is:


