An electron of energy 10.8 ev is incident on an H atom then?
- the electron will come out with 10.8 ev energy
- the electron will completely absorbed
- 10.2 ev will be absorbed and it will come out with 0.6 ev energy
- none
- the electron will come out with 10.8 ev energy
- the electron will completely absorbed
- 10.2 ev will be absorbed and it will come out with 0.6 ev energy
- none
1 Answer
Feb 26, 2018
That is way more energy than necessary to make
A hydrogen atom has an electron affinity of
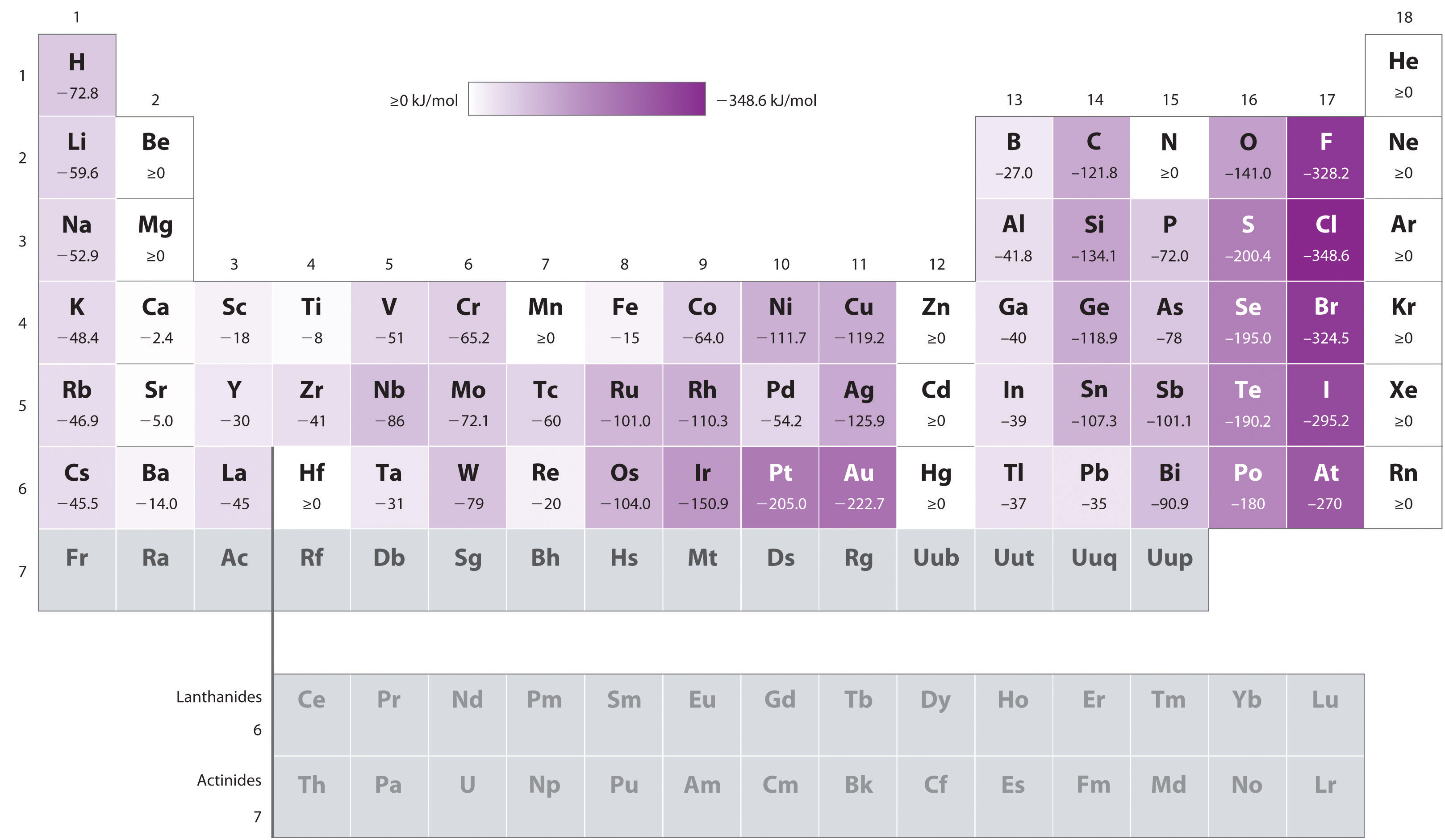 http://2012books.lardbucket.org/
http://2012books.lardbucket.org/
As a result, by accepting an electron,
"H"(g) + e^(-) -> "H"^(-)(g)
the electron is stabilized by
"10.80 eV" - "0.755 eV" = "10.05 eV"
to use. As the electron affinity of
"10.05 eV" - "0.755 eV" = "9.29 eV"
of energy. So... none of the above.

