An electron will have highest energy in which set ? A. 3,2,1,1/2 B. 4,2,-1,1/2 C. 4,1,0,-1/2 D. 5,0,0,1/2
2 Answers
B. 4, 2, -1,
Explanation:
Greater the
If two electrons have same
A.
#(n + l) = (3 + 2) = 5# B.
#(n + l) = (4 + 2) = 6# C.
#(n + l) = (4 + 1) = 5# D.
#(n + l) = (5 + 0) = 5#
Therefore, B is the correct option.
We must identify the orbital first...
Then we would find that the
More info on quantum numbers is already given here.
This indicates a
#3d# orbital of some sort, with a spin-up electron.
This indicates a
#4d# orbital of some sort, with a spin-up electron.
This indicates a
#4p_z# orbital, with a spin-down electron.
This indicates a
#5s# orbital, with a spin-up electron.
Data from Inorganic Chemistry (Miessler, Fischer, and Tarr) shows that the
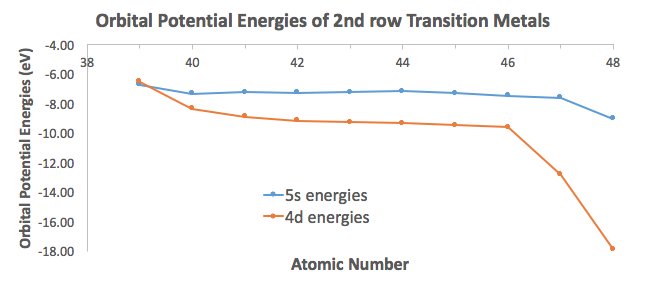
Only yttrium apparently has


