Are binary ionic compounds composed of metals and nonmetals, typically from opposite sides the periodic table?
1 Answer
Mar 14, 2017
Most of the time.
Explanation:
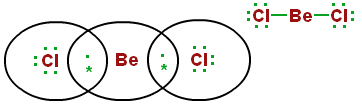
Usually a metal and a nonmetal from opposite sides of the periodic table form an ionic bond. However, there are exceptions. For example, beryllium, which is an alkaline earth metal in group 2 of the periodic table. The halogens in group 17 bond with beryllium to produce beryllium halides, such as beryllium chloride. Instead of forming an ionic bond, they form covalent bonds, which are molecular. This is possible because Be is stable with four valence electrons.
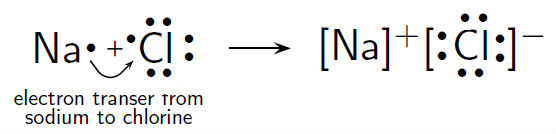
Typical binary ionic compound: NaCl
Beryllium chloride