Are gas molecules assumed to exert no forces of attraction or repulsion on each other?
1 Answer
Mar 7, 2018
Usually, but it's not true.
Explanation:
Many times, when we're doing calculations on gases, we assume that they're an ideal gas.
An ideal gas is a gas that basically follows the Kinetic Molecular Theory, and one postulate of the Kinetic Molecular Theory assumes that gas particles don't attract or repel each other.
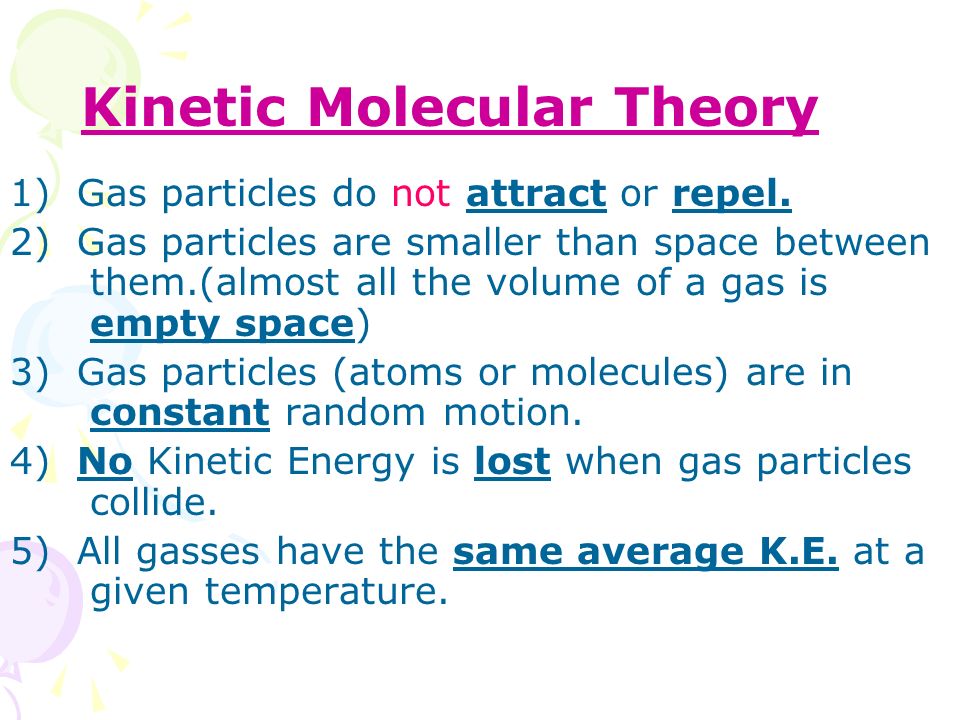
However, this isn't true—in real gases, intermolecular forces are present.

