Arrange the size of Na, Na+ ,Cl,Cl- in increasing order?
1 Answer
So how do we define size.....?
Explanation:
And the only reasonable definition is by ATOMIC or IONIC RADIUS. We know that (i) atomic radius decreases ACROSS a Period from left to right as we face the Table; and (ii) atomic radius INCREASES down a Group, a column of the Periodic Table. Why?
Well, atomic size depends on electronic radius, and INCOMPLETE electronic shells shield the nuclear charge very ineffectively. And thus across the Period atomic size decreases. Once the shell is full, however, shielding of nuclear charge becomes relatively effective, and the next Period builds on a new valence shell that is farther removed from the nucleus.
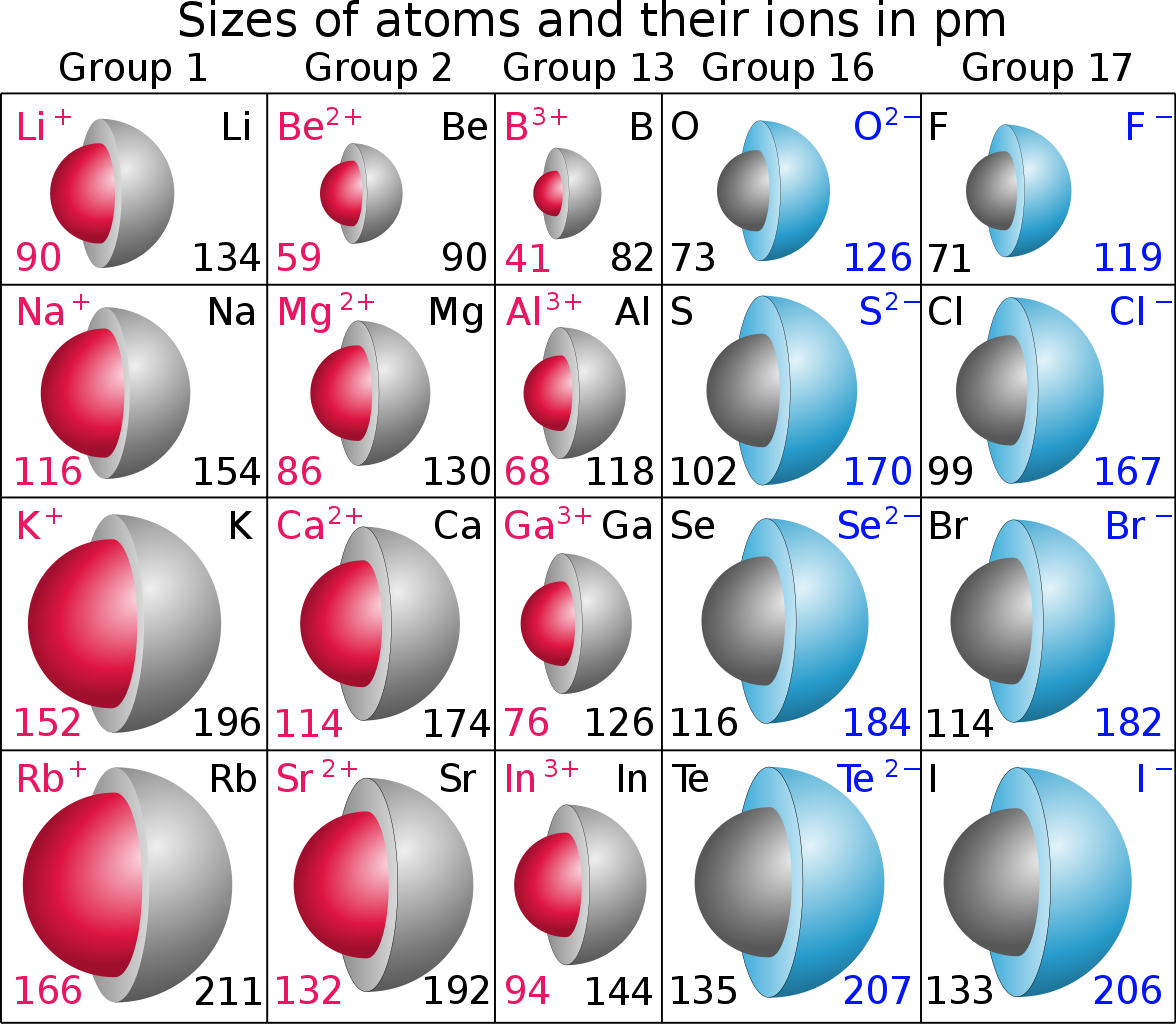
The numbers are in
On the other hand ionic radius depends on the nature of the element: metal or non-metal. Metals are electron rich and REDUCING, and form positively charged cations; and thus, since the valence electrons are removed, their ions should be of reduced radius. Does the illustration support this?
On the other, other hand, non-metals are oxidizing, that is they accept electrons to form negatively charged ions... And since they accept electrons, the ionic radius of the ANION, should be LARGER than the parent atom. Does the diagram support this conjecture? Why or why not?
And in fact many Periodic properties may be rationalized on this contest between nucular charge, and shielding by other electrons.
And so to your question, finally!

