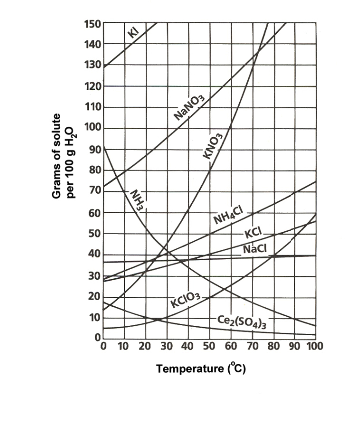
At room temperature (approximately 20-25 degrees celsius) , which compound is the least soluble and why?
1 Answer
Jun 17, 2018
Would it not be the chlorate salt OR the cerium salt....under the given conditions....
Explanation:
We take the
If you want to win brownie points ask your teacher why the solubility of cerous sulfate DECREASES with increasing temperature....this is a VERY unusual phenomenon, and I bet that your teacher does not give you a satisfactory answer...

