Based on the general trends of successive ionization energies, which of the following statements is correct?
Hello all, just a chemistry question here:
Based on the general trends of successive ionization energies, which of the following statements is correct? (multiple statements may be selected)
- Nitrogen has a larger first ionization energy than Boron
- Silicon has a smaller second ionization energy than Carbon
- Strontium has a larger third ionization energy than Rubidium
- Magnesium has a smaller fourth ionization energy than Calcium
I mostly wanted to find out if the only way to solve this is by writing out each electron configuration to the appropriate "steps" (as in, second, third, fourth, etc. ionization energies) or if there is a more efficient way to do this. Would I be able to figure these out just by looking at the periodic table? Or some other more useful method? However you solve it, please write out all steps as I want to understand how to do this with different elements as well as the ones listed in the problem above. Thank you in advance!
Hello all, just a chemistry question here:
Based on the general trends of successive ionization energies, which of the following statements is correct? (multiple statements may be selected)
- Nitrogen has a larger first ionization energy than Boron
- Silicon has a smaller second ionization energy than Carbon
- Strontium has a larger third ionization energy than Rubidium
- Magnesium has a smaller fourth ionization energy than Calcium
I mostly wanted to find out if the only way to solve this is by writing out each electron configuration to the appropriate "steps" (as in, second, third, fourth, etc. ionization energies) or if there is a more efficient way to do this. Would I be able to figure these out just by looking at the periodic table? Or some other more useful method? However you solve it, please write out all steps as I want to understand how to do this with different elements as well as the ones listed in the problem above. Thank you in advance!
1 Answer
You should be able to make a good assessment just by looking at the Periodic Table............
Explanation:
Ionization energies INCREASE across a Period, from left to right as we face the Table, BUT DECREASE down a Group. And most of the time we can rationalize this on the basis of very simple electrostatic arguments. (Sometimes, but not always, we would have to use arguments based on electronic structure.)
Unfilled electronic shells shield the nuclear charge very INEFFECTIVELY. The result is that ACROSS the Period from left to right, ionization energies increase markedly.
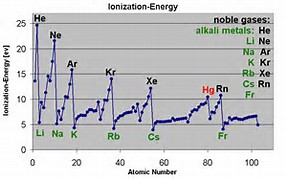
That the Noble gases have the highest first ionization energy in their given Period is the evidence we draw on to make that assessment.
As we assess SUCCESSIVE ionization energies, we would assume it would be harder a priori to remove an electron, and and second ionization energies should rise markedly (and indeed they do, especially if we have to remove an electron from a non-valence, inner core, electronic shell).
And thus we bear these points in mind when we try to answer the questions you have posed.
For nitrogen,
For silicon,
For strontium,
For magnesium versus calcium, we would presume that nuclear charge dominates, and thus calcium,
Anyway, I urge you to look at actual data. And also look at the relevant chapter in your inorganic text.

