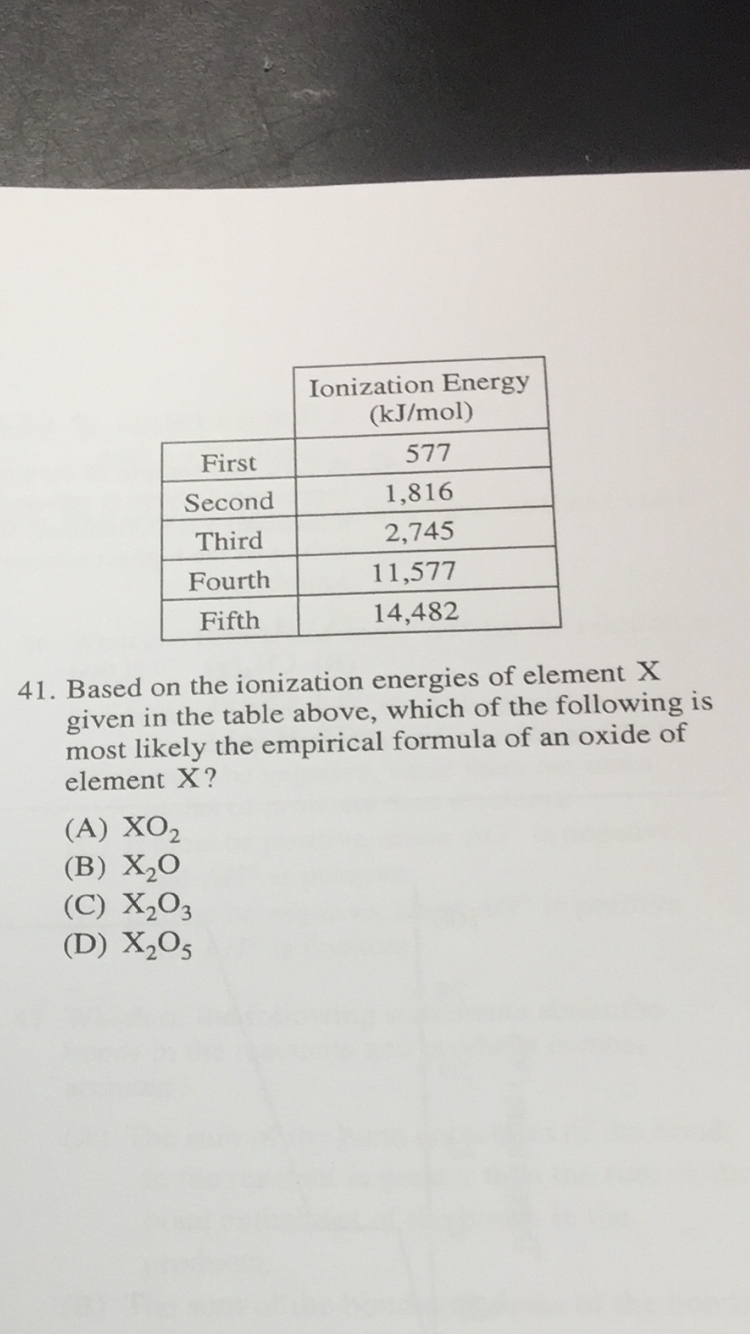
Based on the ionization energies of element X given in the table, which of the following is most likely the empirical formula of an oxide of element X?
1 Answer
Explanation:
From the ionization energy table it is likely that the element X has three valance electrons. The first three ionization energy are relatively low. There is a large jump to the fourth ionization energy. This data indicates that there are only three electrons in the outer shell. Removing electrons from an inner shell requires much more energy.
The three valance electrons will be lost to create a stable electron structure. Losing three valance electrons will create a +3 charge.
Compounds have a net electric charge of zero
X has a charge of +3 and Oxygen has a charge of -2.
So
The Least Common Multiply of 3 and 3 is 6.
so the formula would be
