Order pyrrolidine and piperidine by basicity?
which is more basic , 1st or 2nd ?
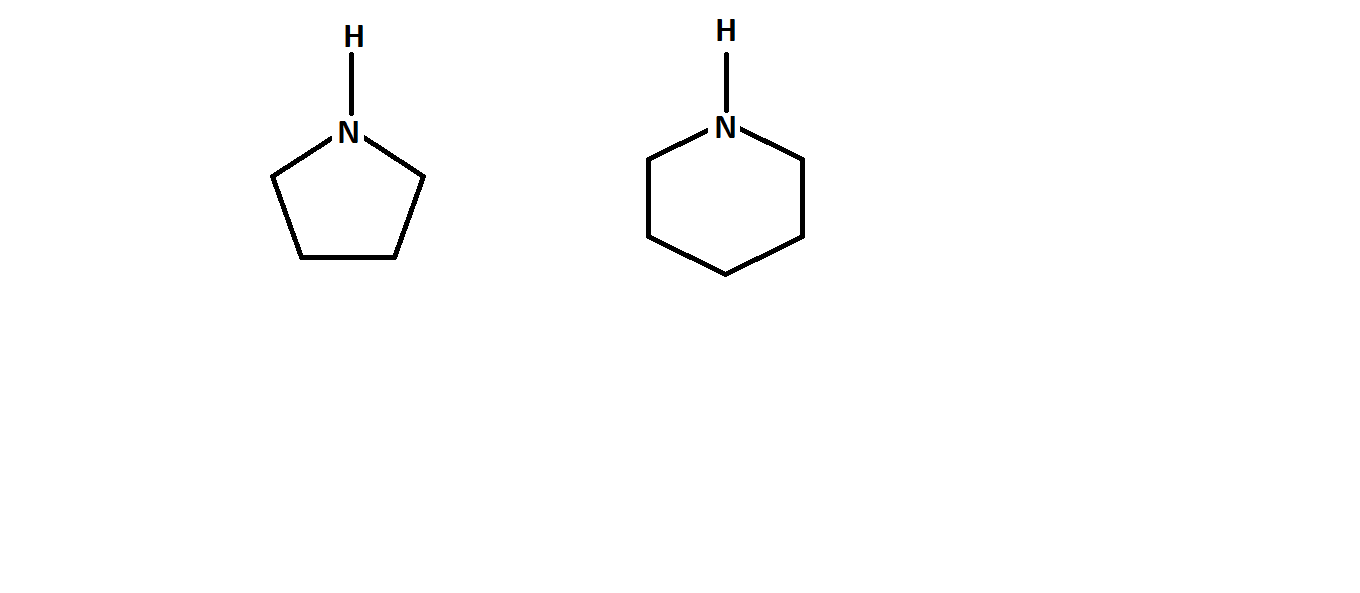
which is more basic , 1st or 2nd ?

1 Answer
Sep 10, 2017
Hardly any difference... but pyrrolidine is SLIGHTLY more basic by
And one would not know that ahead of time... The
And it boils down to pyrrolidine being a five-membered ring. Apparently, it gains a touch more conformational stabilization after protonation by slightly twisting away from its envelope conformation.
And since its conjugate acid is SLIGHTLY more stable, pyrrolidine is a SLIGHTLY stronger base.

