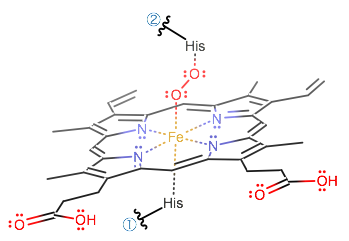
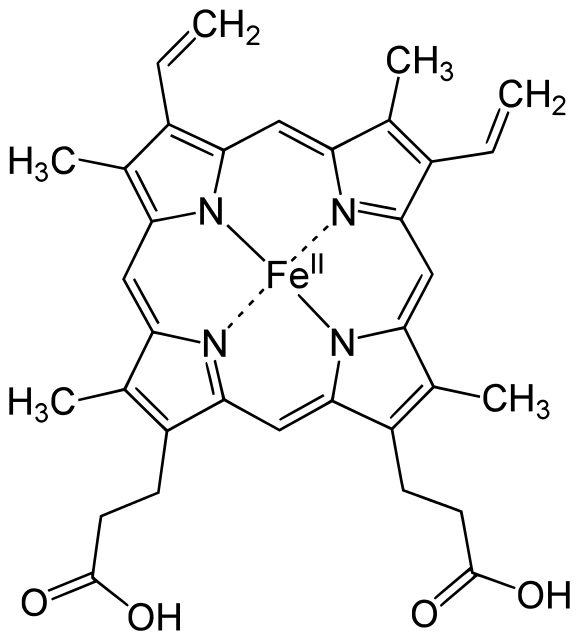
Being given the structure of hemoglobin, determine the hybridization state of the iron atom?
1 Answer
Jan 28, 2018
Since the iron has no lone pairs of electrons, AND only four electron groups, there can only be four pure atomic orbitals used in this hybridization.
We need bonding on the
- An
#bbs# orbital is always needed, regardless of what atom is at the center. - A
#bb(p_x)# and#bb(p_y)# orbital are needed to bond on the#xy# plane. - A
#bb(d_(x^2-y^2))# orbital is also needed to bond on the#xy# plane (its lobes are oriented along the axes), but it mainly provides the same orbital phase along each axis. - A
#p_z# and#d_(z^2)# are NOT NEEDED YET because the#z# axis has no atoms. - A
#d_(xy)# cannot be used because it is aligned between the axes for#pi# bonding, and hybridization only occurs to make#sigma# bonds.
Thus, we have
#4s + 4p_x + 4p_y + 3d_(x^2-y^2) -> 4(sp^2d)#
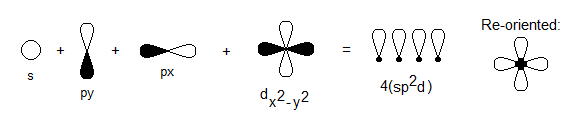
This is true for square planar complexes , and you should always think about the orbitals and their geometries when deciding what orbitals are used.
(And so, this could not have been
Note: since we often talk about hybridization even if the bonds have not been made yet, and hemoglobin iron can make six bonds total,