Between pi bond and sigma bond, which bond can be broken easily and why?
1 Answer
Oct 2, 2014
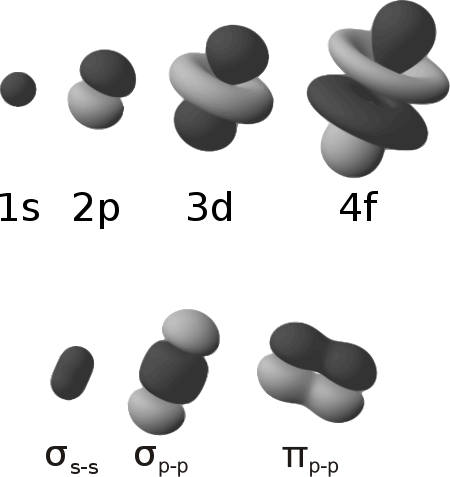
By this definition, common forms of sigma bonds are s+s, pz+pz, s+pz and dz2+dz2.
From the perspective of quantum mechanics, this bond's weakness is explained by significantly less overlap between the component p-orbitals due to their parallel orientation.
More about:

