Buffer Solution Help ?????
1 Answer
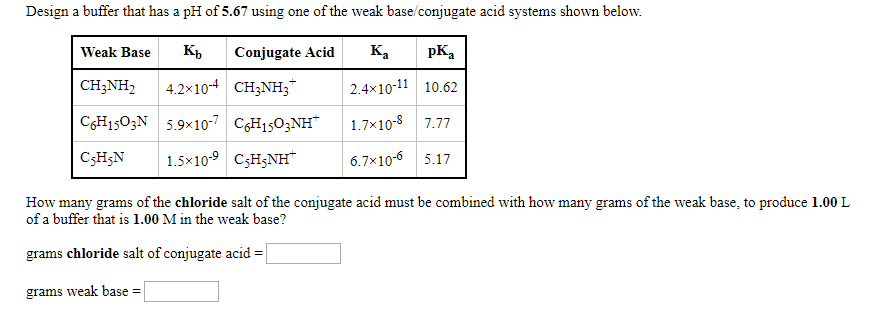
We must dissolve 78.1 g of the weak base (pyridine) and 69.5 g of pyridinium chloride in enough water to make 1 L of solution.
Explanation:
The best buffer is one in which
The acid with the closest
The chemical equation for the equilibrium is
#"C"_5"H"_5"NH"^"+" + "H"_2"O" ⇌ "C"_5"H"_5"N" + "H"_3"O"^"+""#
Let's rewrite this equation as
#"BH"^"+" + "H"_2"O" ⇌ "B" + "H"_3"O"^"+"#
We can apply the Henderson-Hasselbalch equation:
#color(blue)(bar(ul(|color(white)(a/a)"pH" = "p"K_text(a) + log((["B"])/(["BH"^"+"]))color(white)(a/a)|)))" "#
So, we want 1 L of a buffer that contains 1.00 mol of

