By adding 15 g of KCl to 25 g of H2O is the solution saturated or unsaturated?
Just the answer
Just the answer
1 Answer
Apr 7, 2018
OK. Just the answer: saturated.
Explanation:
I'm guessing that your instructor has asked you to get the answer from a chart k like the one below,
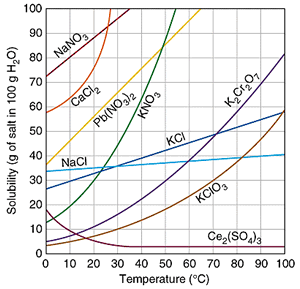
We see from the chart that the solubility of
Thus, if you add 15 g of

