Calculate the ΔH reaction?
CO(g) + 1/2O2(g) -----> CO2(g) ∆H = -282 kJ
C(s) + O2(g) -----> CO2(g) ∆H = -393 kJ
CO(g) ---> C(s) + 1/2O2(g) ∆H = ? kJ
I got an answer of -111 KJ but the answer is +111.. could somebody explain why? thanks
CO(g) + 1/2O2(g) -----> CO2(g) ∆H = -282 kJ
C(s) + O2(g) -----> CO2(g) ∆H = -393 kJ
CO(g) ---> C(s) + 1/2O2(g) ∆H = ? kJ
I got an answer of -111 KJ but the answer is +111.. could somebody explain why? thanks
1 Answer
See below:
Explanation:
Hess' Law tells us that the overall enthalpy change of a reaction is independent of the route taken.
We can set up a Hess Cycle:
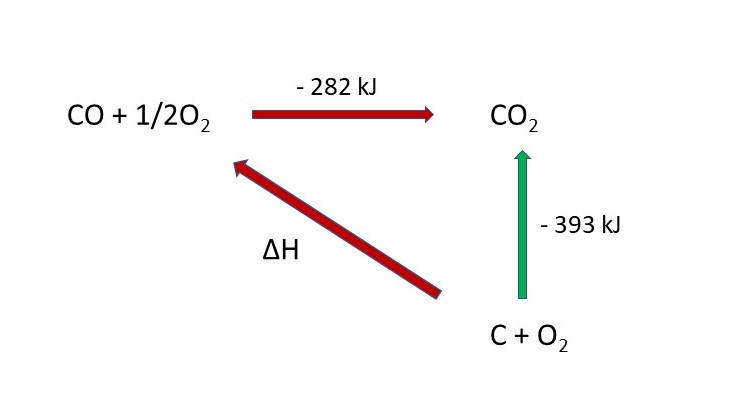
Applying Hess' Law you can see that, in terms of entalpy, the red route must equal the green route. This is because the arrows start and finish in the same place.
This gives us:
However this gives us
We want
This means we must reverse the sign of

