Calculating the heat of the reaction?
1 Answer
Based on the similarities between the given reactions and the reaction of interest, you are being asked to apply the state function property of enthalpy via Hess's Law.
Basically, the idea is that with enthalpy calculations, because enthalpy is a "state function", it only matters what your starting point and ending point are, and not what happens in between.
Hess's Law essentially states that you can manipulate a reaction as follows:
- Scale the reaction
#-># scale the enthalpy value - Reverse the direction of the reaction
#-># reverse the sign of the enthalpy
And however you manipulate it, you preserve the validity of your answer.
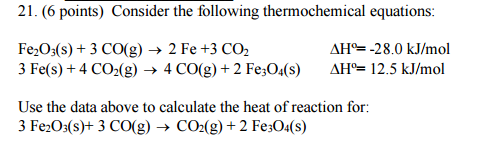
Your reactions to be used are:
(1)
#"Fe"_2"O"_3(s) + 3"CO"(g) -> 2"Fe"(s) + 3"CO"_2(g)# ,#Delta"H"_1^@ = -"28.0 kJ/mol"# (2)
#3"Fe"(s) + 4"CO"_2(g) -> 4"CO"(g) + color(Red)(2)"Fe"_3"O"_4(s)# ,#Delta"H"_2^@ = "12.5 kJ/mol"#
And you are to match this result with the above tabulated reactions:
(3)
#3"Fe"_2"O"_3(s) + color(red)(3)"CO"(g) -> "CO"_2(g) + 2"Fe"_3"O"_4(s)#
Notice how the final result does not have solid iron (
That's all you should need to balance this, because it's such a specific set of scalars to use (
But something's wrong here... This is NOT balanced.
(2)
#3"Fe"(s) + 4"CO"_2(g) -> 4"CO"(g) + color(red)(2)"Fe"_3"O"_4(s)#
Shouldn't this be...
(2)
#color(green)(3"Fe"(s) + 4"CO"_2(g) -> 4"CO"(g) + "Fe"_3"O"_4(s))# ?
From here, we would then compare the new, correct reactions. (The enthalpies are correct enough.)
(1)
#"Fe"_2"O"_3(s) + 3"CO"(g) -> 2"Fe"(s) + 3"CO"_2(g)#
(2)#3"Fe"(s) + 4"CO"_2(g) -> 4"CO"(g) + "Fe"_3"O"_4(s)#
The number of
(3)
#color(green)(3"Fe"_2"O"_3(s) + "CO"(g) -> "CO"_2(g) + 2"Fe"_3"O"_4(s))# ?
Then this works out nicely.
#3("Fe"_2"O"_3(s) + 3"CO"(g) -> cancel(2"Fe"(s)) + 3"CO"_2(g))#
#2(cancel(3"Fe"(s)) + 4"CO"_2(g) -> 4"CO"(g) + "Fe"_3"O"_4(s))#
#"----------------------------------------------------------"#
#color(blue)(3"Fe"_2"O"_3(s) + "CO"(g) -> "CO"_2(g) + 2"Fe"_3"O"_4(s))#
Convince yourself that the number of iron, carbon, and oxygen atoms are balanced. :)
Now simply scale the enthalpies accordingly.
#color(blue)(Delta"H"_"rxn"^@ )= 3Delta"H"_1^@ + 2Delta"H"_2^@#
#=# #color(blue)("Insert your answer here")#

