Can anyone explain the reaction mechanism below with a diagram of the movement of electrons?
1 Answer
May 12, 2018
How about this?
Explanation:
Step 1. Nucleophilic substitution
The reaction is base-catalyzed, so the nucleophile is the naphthoxide ion. I will use
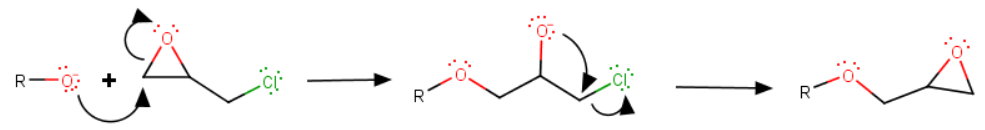
The α-naphthoxide ion attacks the less-substituted substituted carbon atom of the oxirane ring to form an alkoxide ion.
The new ion, in turn, attacks the carbon adjacent to the
Step 2. Nucleophilic substitution
The isopropylamine attacks the less substituted carbon atom of the oxirane ring.
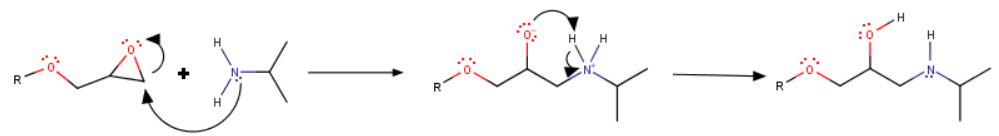
During workup, the oxide and ammonium ions become protonated/deprotonated to give the amino alcohol.

