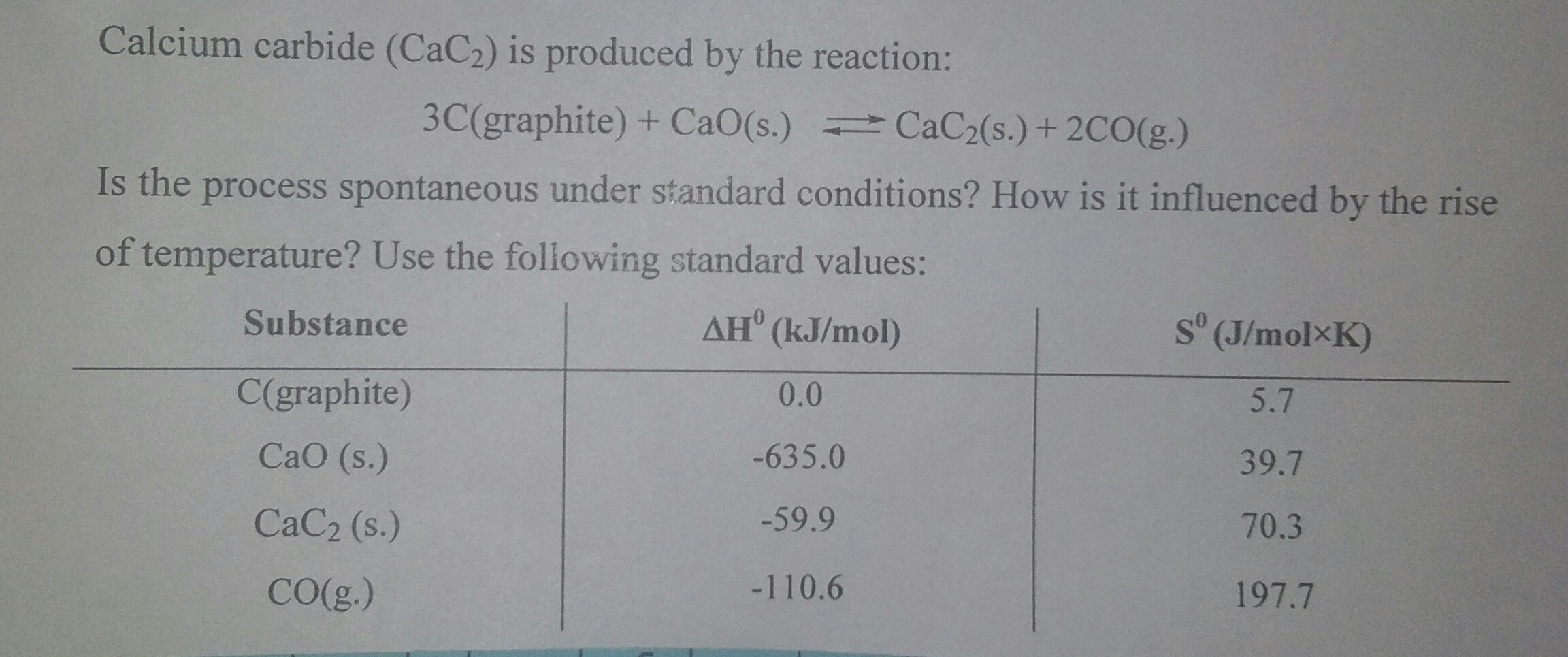
Can anyone solve my this chemistry work?
1 Answer
Not spontaneous at standard conditions, and an increase in temperature will make the reaction more likely to become spontaneous, becoming spontaneous when
Explanation:
A reaction is spontaneous if
The reaction is not spontaneous in standard conditions.
An increase in temperature will result in a greater value for
The reaction becomes spontaneous when
When

