Can ferric(3) oxide or ferric(3) acetate be substituted with ferric(3) chloride for phenol test ?
Can ferric(3) oxide or ferric(3) acetate be substituted with ferric(3) chloride for phenol test ?
Can ferric(3) oxide or ferric(3) acetate be substituted with ferric(3) chloride for phenol test ?
1 Answer
Dec 17, 2017
I don't think ferric oxide and ferric acetate can be substituted with
Explanation:
The reaction between phenol and ferric chloride is
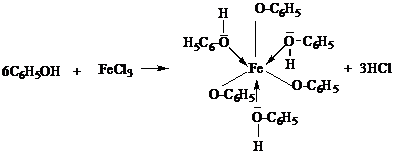
Then, why do I think ferric oxide and ferric acetate is inappropriate?
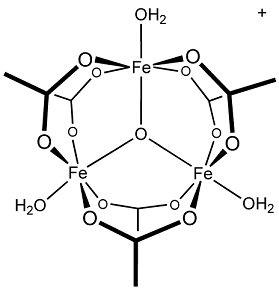
・Ferric oxide(
・Ferric acetate(