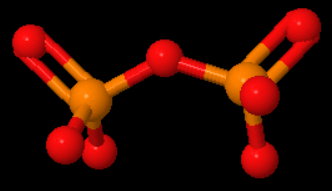
Can someone explain to me what the hybridization and molecular geometry for p2O7-4 would be considered?
1 Answer
Here's what I get.
Explanation:
Step 1. Draw the Lewis structure
There are three central atoms:
Arrange them symmetrically in the order:
Attach the remaining
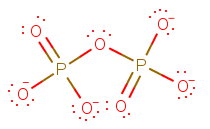
Step 2. Use VSEPR theory to determine the electron geometries
There are four electron domains around each central atom.
This corresponds to tetrahedral electron geometries and
Step 3. Determine the molecular geometries
Each
The molecular geometry around each
The central
This corresponds to a bent molecular geometry.
The shape of the pyrophosphate ion is