Can someone help me with this question?
1 Answer
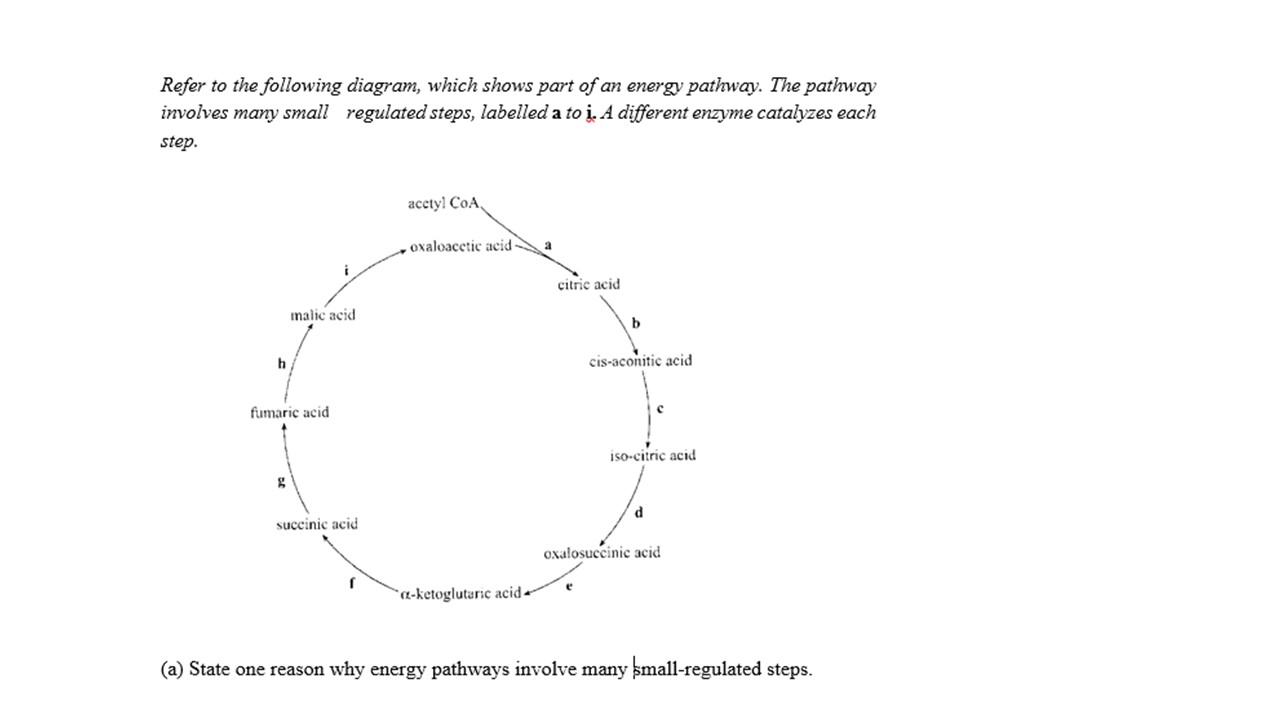
To reduce the level of heat energy release at any one step.
Explanation:
The burning of sugar is a highly exothermic reaction.
A fun experiment is to take powered or finely ground sugar and drop it on a very hot burner. The sugar will burst into flames even before it touches the burner. ( Have the students wear safety glasses)
If the oxidation of sugar in the living cell took place in one unregulated large reaction the temperature produced would be so high the living cell would no longer be a living cell.
By breaking the overall chemical reaction into smaller well regulated steps or stages the temperature produced at any one step is small enough not to disrupt or destroy cell processes or structures.
The total heat energy produced by one large reaction is the same as the heat energy produced by a series of small well regulated small reactions but the temperature or level of heat is different.
Also the energy release is controlled and much of the energy is not released as heat at all. The energy released by the oxidation of the sugar is converted to chemical bond energy stored in ATP molecules. The energy in the ATP can be used to produce other chemical reaction in the cell as needed besides heat.
The mitochondria regulates the oxidation of the sugar molecules. The final step where the Hydrogen ions and combined with Oxygen to produce ATP occurs in the the inner membrane of the mitochondria so that the rest of the cell is protected.
