Can someone please help me with this Question?
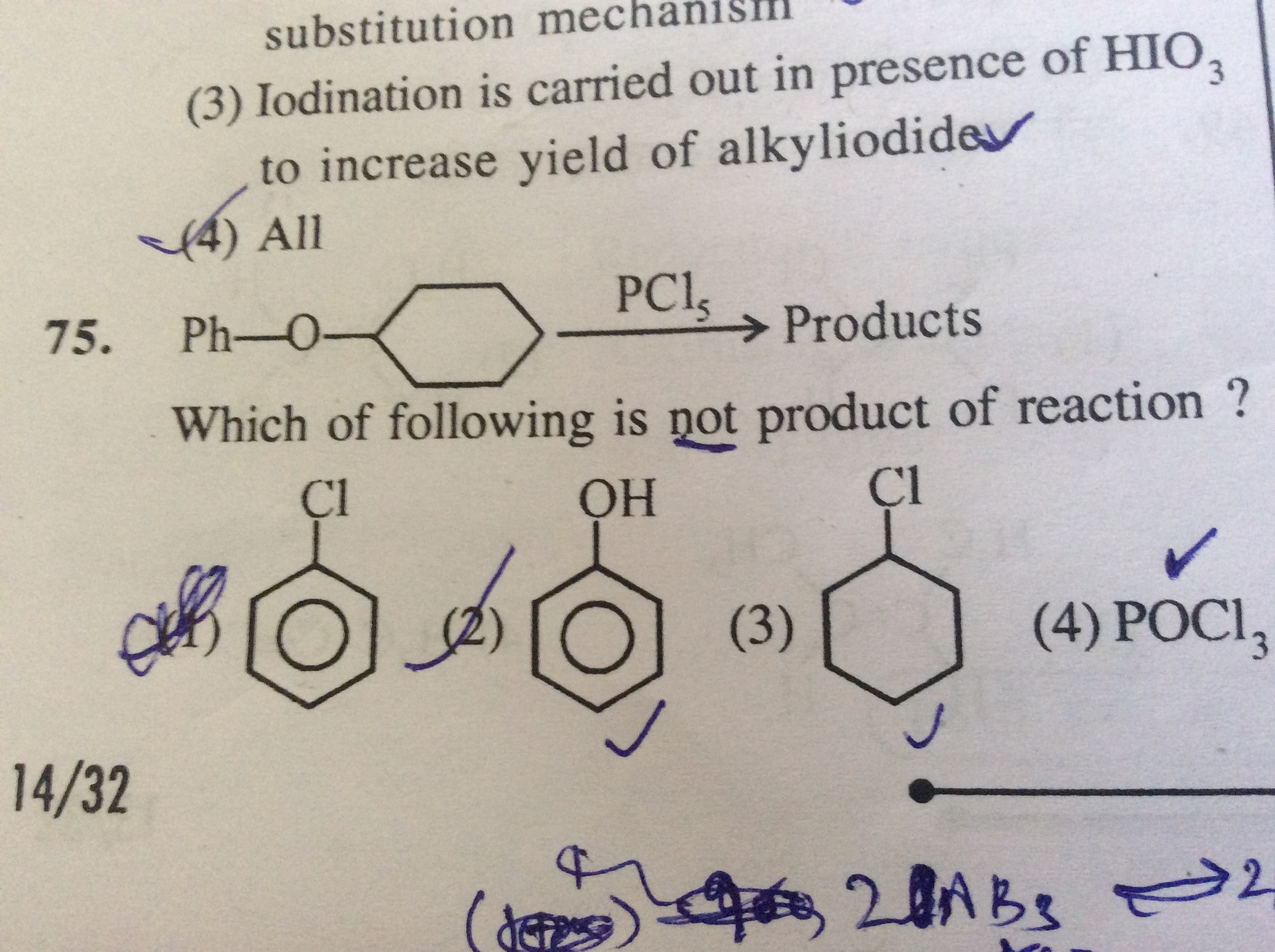
1 Answer
Apr 24, 2018
The correct answer is (1).
Explanation:
Thus, the reaction with cyclohexyl phenyl ether gives chlorocyclohexane and phenol.
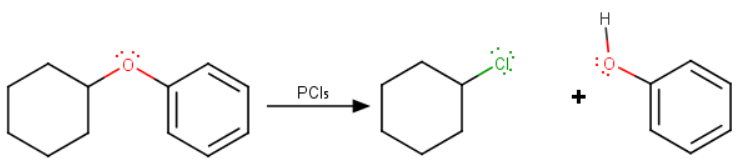
The mechanism probably involves an
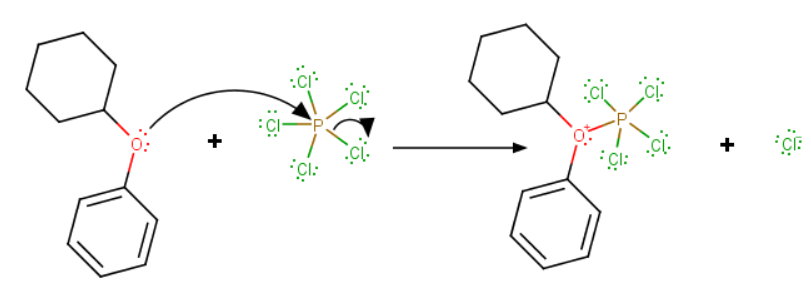
Then, in a second
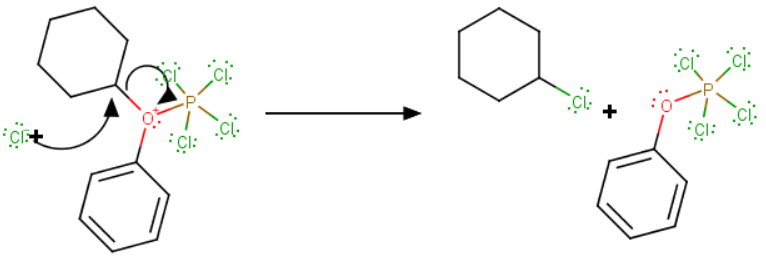
Aqueous workup hydrolyzes the aromatic phosphorus compound to phenol and

