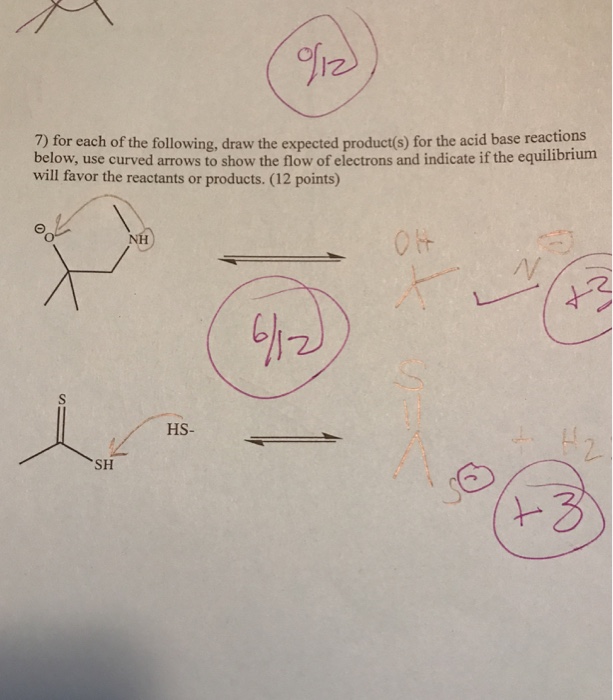
Can you please solve this question ?
2 Answers
Well, for the first scenario you gots a alkoxide, and a secondary amine...
Explanation:
...and the following equilibrium may occur...
...we would expect the equilibrium would lie to the LEFT, as we face the page. Alcohols are more acidic than amines...
And for the second reaction we gots another acid base reaction...
..again, we would expect the equilibrium would lie to the LEFT, as we face the page.
I. Reactants; II. Products
Explanation:
Part I
The negatively-charged
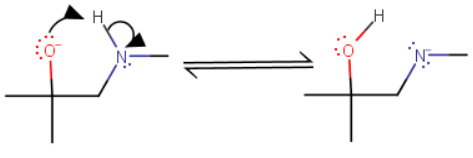
An alcohol is a stronger acid than an amine, so an alkoxide ion is a weaker base than an amide ion.
Since the amide ion (on the right) is the stronger base, the alkoxide (on the left) is the major product.
The position of equilibrium favours reactants.
Part II
The dithioacetic acid reacts with the hydrogen sulfide ion, forming dithioacetate ion and hydrosulfuric acid.
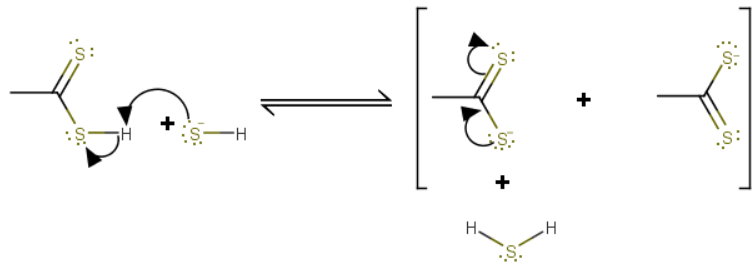
The dithioacetate ion is the more stable product because it has two equivalent resonance forms.
The position of equilibrium favours products.


