Can you use Le Chatelier's principle explain the role of OH ions in the CrO₄²⁻ ⇌ Cr₂O₇²⁻ equilibrium?
1 Answer
Le Châtelier's Principle explains the role of hydroxide ions in the chromate/dichromate equilibrium.
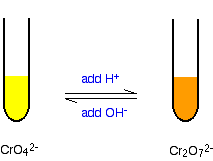
Solid potassium dichromate K₂Cr₂O₇ dissolves in water to form an orange solution.
The orange dichromate ion is in equilibrium with the yellow chromate ion, as in the equation:
Cr₂O₇²⁻ + H₂O ⇌ 2 CrO₄²⁻ + 2 H⁺
orange ⇌ yellow
This is a dynamic equilibrium.
Le Châtelier's Principle states that when a stress is applied to a system at equilibrium, the system will respond in such a way that it relieves the stress.
See What is Le Chatelier's principle?.
Thus, changing the concentration of H⁺ will affect the position of equilibrium.
If we add OH⁻ ions, they will react with the H⁺ ions to form water.
The system will respond by forming more H⁺ ions (and also more CrO₄²⁻ ions). The position of equilibrium moves to the right, and the colour changes from orange to yellow.
If we remove OH⁻ ions by adding H⁺, the system will respond by forming removing H⁺ ions (and also CrO₄²⁻ ions).
The concentration of Cr₂O₇²⁻ ions will increase. The position of equilibrium moves to the left, and the colour changes from yellow to orange.