Can you walk me through simple dilutions of 1 M HCL acid? We have 1 Liter of 1 M HCL. I have 5 pieces of metal each about 4 grams. I have 5 containers. One will be all HCL and a control will be all distilled water. I need 75%, 50%, 25% and 10% solutions
So I guess that is where I am confused. My son is making up this experiment for science. We can define the volumes. We have 1 liter of HCL 1 molar solution. The goal is to show how the metal changes in different % of hydrochloric acid....
So I guess that is where I am confused. My son is making up this experiment for science. We can define the volumes. We have 1 liter of HCL 1 molar solution. The goal is to show how the metal changes in different % of hydrochloric acid....
1 Answer
May 15, 2018
You can create a table like this:
Explanation:
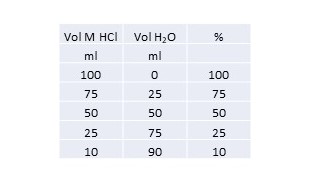
I am assuming that you are investigating the effect of concentration on the rate of a reaction.
I have assumed that the scale here means that 100% = 1M.
For your 1st run you take 100 ml of the 1M HCl.
For your 2nd run you take 75 ml and add 25 ml of water to make up 100 ml.
And so on.
The total volume is always 100ml.
Your control will be 100 ml of distilled water.
If you need a bigger total volume, say 200 ml, just double all the quantities.

