Complete the following statement: Water (H2O) is a?
- salt.
- strong electrolyte.
- flammable solvent.
- polar solvent.
- linear molecule.
- non-polar solvent.
- salt.
- strong electrolyte.
- flammable solvent.
- polar solvent.
- linear molecule.
- non-polar solvent.
1 Answer
Water
Explanation:
-
salt
The definition of a salt is an ionic compound formed by the neutralization reaction of acids and bases.
Water is not a salt. -
strong electrolyte
An electrolyte is an substance that produces ions when dissolved in water, so water is not an electrolyte. -
flammable solvent
Solvent is something that a solute dissolves in, so water is a solvent. However, water is not flammable, as it extinguishes fire rather than setting it. -
polar solvent
This is TRUE . Again, water is a solvent, and it is also polar. Here is the Lewis structure below:
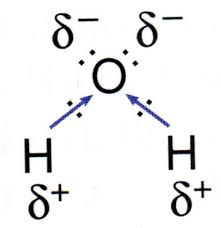
Oxygen is much more polar than both the hydrogen cations combined, meaning that water is polar. -
linear molecule
Based on the Lewis structure above, we can see that there are two lone pairs on oxygen. This molecular geometry of water is bent, meaning that it is not a linear molecule. -
non-polar solvent
As said earler, water is polar, so this is incorrect.
Therefore, water
Hope this helps!

