Compound Y is a hydrocarbon that contains 85.7% carbon by mass. The diagram shows mass spectrum of compound Y Determine molecular formula of Y?
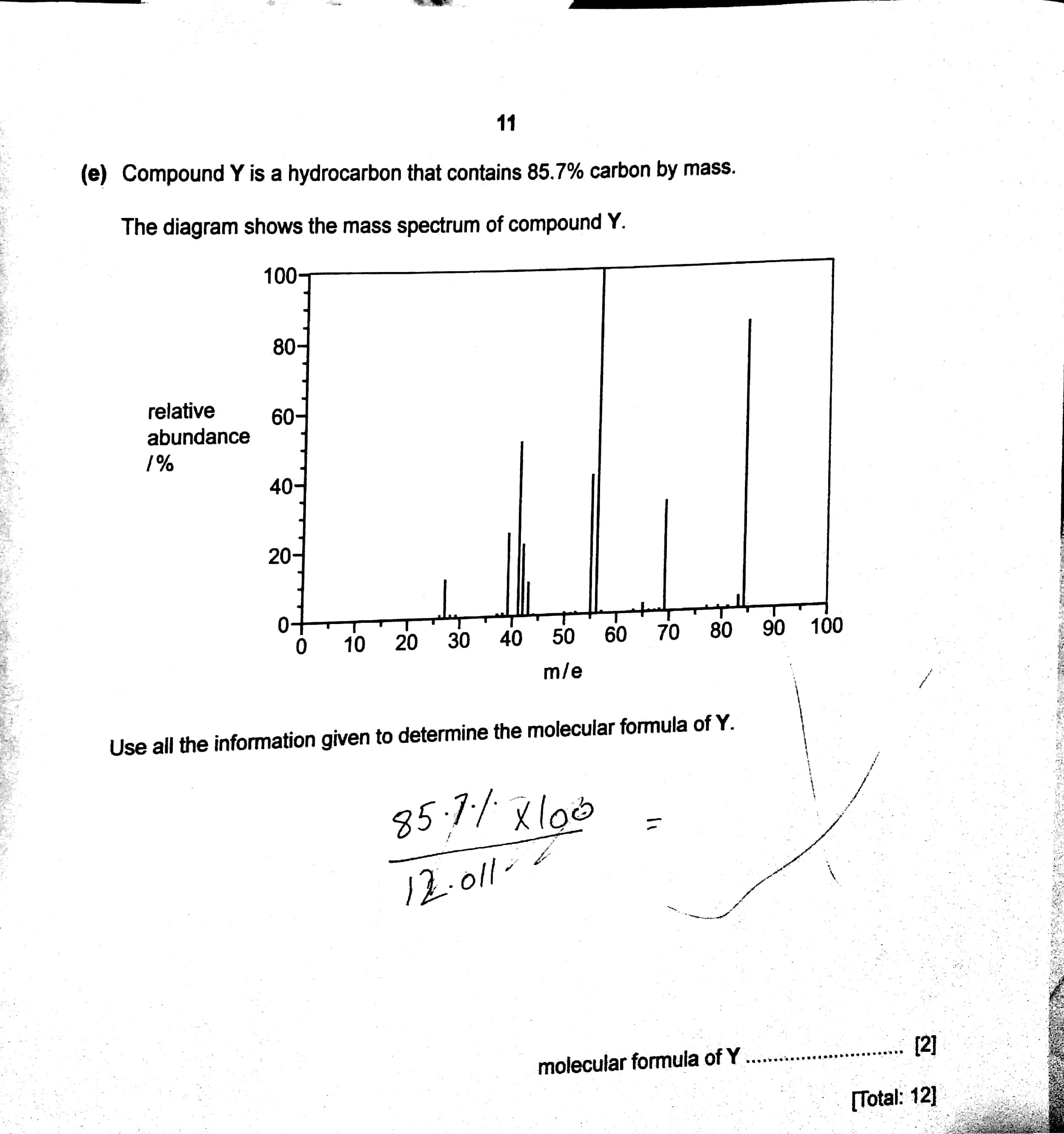
1 Answer
May 5, 2018
I gets...
Explanation:
We know that the hydrocarbon is
Now clearly the empirical formula is
But the molecular formula is a whole number of the empirical formula....and this is....
And so our molecular formula is
Good question!

