Compute normalization constant A if #n=1#?
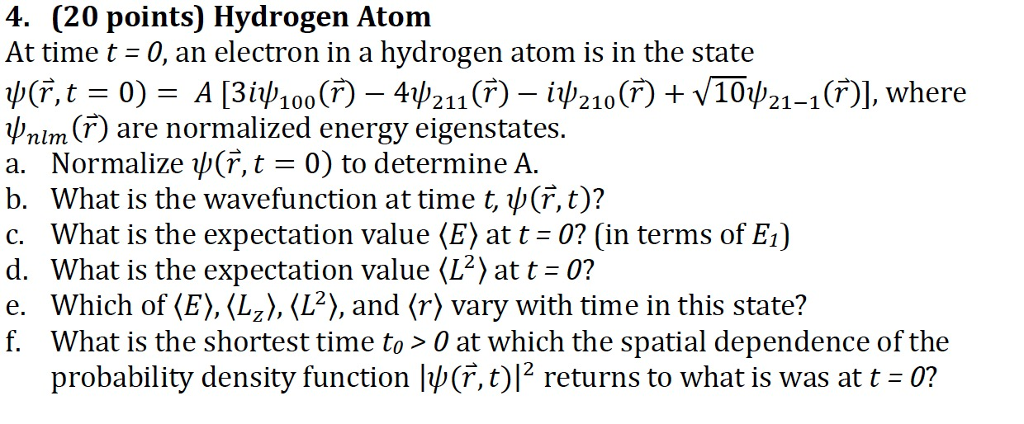
First of all, those are energy eigenvalues same as eigenstates ,Energy is the operator but we do not know the eigenfunction. Are hydrogen energy eigenstates orthonormal?Why?Does this have any effect when we separate ψ(x, t) into spatial wavefunction and time function
I suppose (Q1) is to be solved by the properties of orthonormality and i get an
answer of #1/sqrt(18)# but can it be wrong?
Then i get an answer of #alpha^2muc^(2)4.22/18# for #<E>#
I think the wavefunction remains same at time t because the time part #e^(iomegat)# becomes 1.

First of all, those are energy eigenvalues same as eigenstates ,Energy is the operator but we do not know the eigenfunction. Are hydrogen energy eigenstates orthonormal?Why?Does this have any effect when we separate ψ(x, t) into spatial wavefunction and time function
I suppose (Q1) is to be solved by the properties of orthonormality and i get an
answer of
Then i get an answer of
I think the wavefunction remains same at time t because the time part
3 Answers
This wavefunction, or state function, is constructed as a linear combination of eigenstates that are themselves orthonormal ... if they are not, then nobody would want to do this problem because it would take them days.
CLEARLY,
Regardless, we set it up the easy way as
#??? = |c_1|^2 + |c_2|^2 + ...#
#= (3i)^"*"(3i) + (-4)^2 + (-i)^"*"(i) + (sqrt10)^2#
#36 = 9 + 16 + 1 + 10#
So the magnitude of this wave function is
#=> A = 1/6#
The hard way is to do this...
#1 = A^2int_(0)^(2pi) int_(0)^(pi) int_(0)^(oo)[3ipsi_(100)(r) - 4psi_(211)(r) - ipsi_(210)(r) + sqrt(10)psi_(21-1)(r)]^"*"[3ipsi_(100)(r) - 4psi_(211)(r) - ipsi_(210)(r) + sqrt(10)psi_(21-1)(r)]r^2drsin thetad thetad phi#
Here this is made easier because the wave functions apparently, somehow, are invariant to angular coordinates...
Next, we ignore cross-terms, which are orthogonal, and only obtain the diagonal terms, which are normalized.
#1 = A^2 cdot 4pi int_(0)^(oo)[9psi_(100)^"*"(r)psi_(100)(r) +16psi_(211)^"*"psi_(211)(r) + psi_(210)^"*"(r)psi_(210)(r) + 10psi_(21-1)^"*"(r)psi_(21-1)(r)]r^2dr#
Separate integrals to get:
#= 9A^2 cdot 4pi int_(0)^(oo)psi_(100)^"*"(r)psi_(100)(r) r^2dr + 16A^2 cdot 4pi int_(0)^(oo)psi_(211)^"*"psi_(211)(r)r^2dr + A^2 cdot 4pi int_(0)^(oo)psi_(210)^"*"(r)psi_(210)(r)r^2dr + 10A^2 cdot 4pi int_(0)^(oo)psi_(21-1)^"*"(r)psi_(21-1)(r)r^2dr#
Each of these are normalized after including the
#1 = 9A^2 cdot 1 + 16A^2 cdot 1 + A^2 cdot 1 + 10A^2 cdot 1#
#= A^2(9 + 16 + 1 + 10)#
#= 36A^2#
Therefore,
This is to address the rest of the questions, except
Well, this should be trivial. Keep it simple. You tack on
#color(blue)(Psi(r,t)) = c_1psi_(nlm)(r)e^(-iE_nt//ℏ) + c_2psi_(nlm)(r)e^(-iE_nt//ℏ) + . . . #
#= color(blue)(1/6 [3ipsi_(100)(r)e^(-iE_1t//ℏ) - 4psi_(211)(r)e^(-iE_2t//ℏ) - ipsi_(210)(4)e^(-iE_2t//ℏ)+ sqrt(10)psi_(21-1)(r)e^(-iE_2t//ℏ)])#
We can't say any more because these eigenstates aren't degenerate in Hydrogen atom.
The expectation value is well-known as a form of the Rydberg equation for hydrogen atom:
#E_n = -"13.61 eV" cdot Z^2/n^2#
Wave functions are normalized to an arbitrary
(Otherwise, it would be a huge issue that surely would not have gone unnoticed amongst physical chemists everywhere!)
The Hamiltonian is linear, so
#hatH1/6 [3ipsi_(100)(r) - 4psi_(211)(r) - ipsi_(210)(r) + sqrt(10)psi_(21-1)(r)]#
#= 1/6 [3ihatHpsi_(100)(r) - 4hatHpsi_(211)(r) - ihatHpsi_(210)(r) + sqrt(10)hatHpsi_(21-1)(r)]#
#= 1/6 [3iE_1psi_(100)(r) - 4E_2psi_(211)(r) - iE_2psi_(210)(r) + sqrt(10)E_2psi_(21-1)(r)]#
And since for
#E_1 = -"13.61 eV"#
#E_2 = -"13.61 eV"/4 = E_1/4#
it follows that
#hatHpsi(r) = 1/6 [3icdot4E_1/4psi_(100)(r) - E_1/4 cdot 4psi_(211)(r) - iE_1/4psi_(210)(r) + sqrt(10)E_1/4psi_(21-1)(r)]#
#= color(red)(E_1/4)cdot1/6 [color(red)4 cdot 3ipsi_(100)(r) - 4psi_(211)(r) - ipsi_(210)(r) + sqrt(10)psi_(21-1)(r)]#
#color(red)(ne E_1/4 cdot psi(r))#
This is a hugely problematic question, because you cannot get ONE expectation value.
The eigenvalue of a linear combination is only the same as the individual eigenvalues of each wave function if all of them are degenerate eigenstates.
The eigenvalue of
#hatL^2Y_l^(m_l)(theta,phi) = ℏ^2l(l+1)Y_l^(m_l)(theta,phi)#
Therefore,
#hatL^2psi(r)#
#= hatL^2{1/6 [3ipsi_(100)(r) - 4psi_(211)(r) - ipsi_(210)(r) + sqrt(10)psi_(21-1)(r)]}#
But all of these functions have no angular dependence apparently, so the eigenvalue is just zero.
This is again problematic and rather unphysical, because states with
Hydrogen atom is spherically symmetric, so angular momentum is conserved, i.e. it is a constant of the motion. Energy is presumably conserved; the question stated upfront that each
Only position is in flux over time.
I will answer (c), and (d) - the others have already been answered correctly (and (f) will take too much time to type out).
(c)
(d)
Explanation:
(c) We can calculate the expectation value
which is not really a very difficult task because the
If a wavefunction
then the measurement of
Thus, for the given function, the probability that an energy measurement will yield
Note: the normalization constant is
So, the expectation value of the energy is
Since
(d) It is easy to see that the probabilities of getting the results
So


