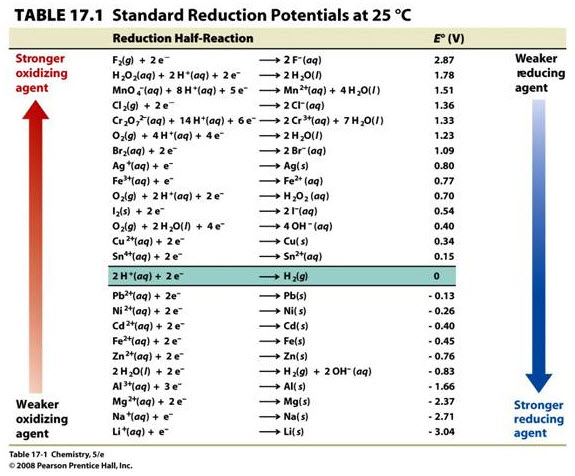
The position of Al in the Elechtro-chemical series shows that Al is stronger reducing agent than Hydrogen. When Al metal is added to dilute #HNO_3#containing higher concentration of # H^+# ions, it reduces the # H^+# ions in the solution to hydrogen gas and itself gets oxidized to #Al(NO_3)_3 #
#2Al(s) + 6 HNO_3(dil)->2Al(NO_3)_3 +3H_2uparrow#
But when Al is dipped in conc . #HNO_3# where the concentration of # H^+# ions is very low, it reduces Nitrogen of #Hstackrel(+5)NO_3# molecule to #NO_2#and itself gets oxidized to #Al_2O_3# which forms very thin invisible protective layer on the metal. It then resists further oxidation of Al atom below the protective layer of #Al_2O_3#.
This occurs when concentrated #HNO_3# is kept in Aluminium container and makes transportation of conc. #HNO_3# in Aluminium container possible.
The equation of possible oxidation reaction occurred during formation of the protective layer of #Al_2O_3#
#2Al(s) + 6 HNO_3(conc)->Al_2O_3(s)+6NO_2(g)+3H_2O(l)#


