Consider the reaction when aqueous solutions of calcium nitrate and potassium hydroxide are combined.?
1 Answer
Feb 20, 2018
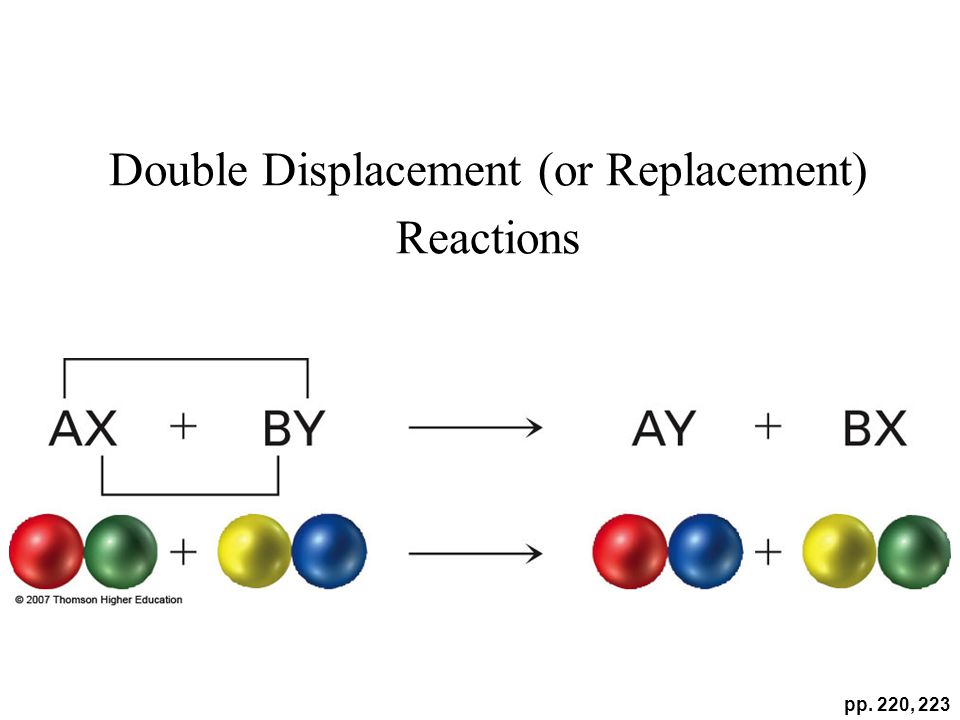
The reaction is a double replacement (displacement) reaction, also called a metathesis.
Explanation:
Balanced equation
The reaction is a double replacement replacement (displacement), also called a metathesis. In a double replacement reaction, cations and anions in the reactants trade places, forming products that include a solid precipitate, an insoluble gas, or water. In this case, one product,
The net ionic equation contains only the ions that participated in the reaction (formation of the precipitate).
The down arrow indicates that the solid is a precipitate.