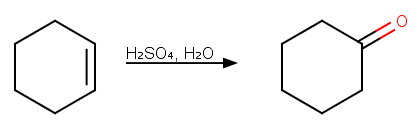
Converting from cyclohexene to this?

What reagents and conditions are required for this?

What reagents and conditions are required for this?
2 Answers
Well, you gots to make a
Explanation:
A tentative synthesis:
How about this?
Explanation:
Step 1. Acid-catalyzed hydration of cyclohexene
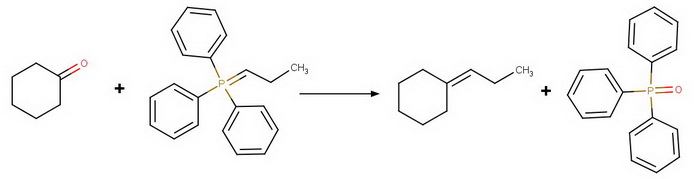
Step 2. Wittig reaction
Then