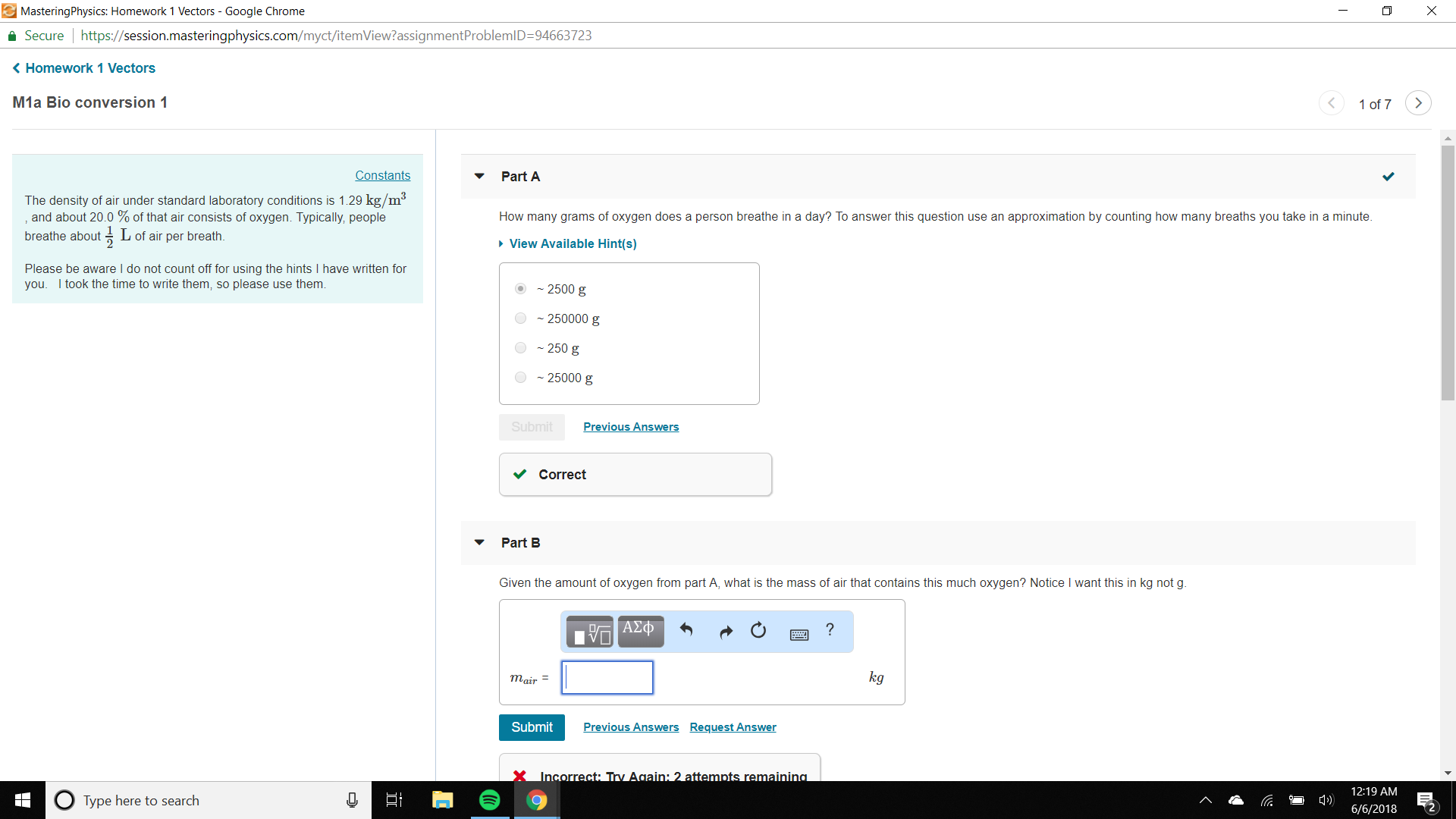
#"B."#
#20%# of air consist of oxygen.
So, mass of air that contains #"2.5 kg"# oxygen is
#"m" = "2.5 kg"/(20%) = "2.5 kg"/20 × 100 = "12.5 kg"#
—————
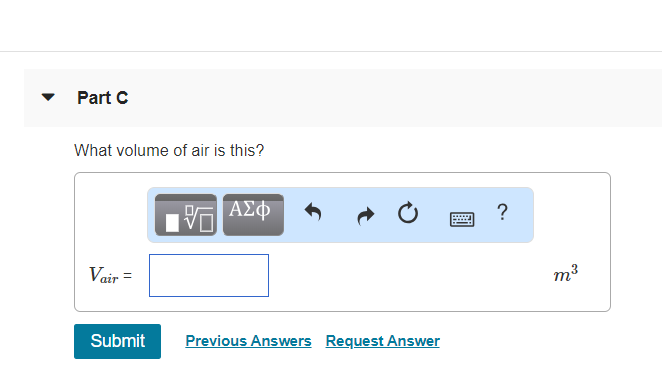
#"C."#
#"V"_"air" = "Mass"_"air"/"Density"_"air"#
#color(white)("V"_"air") = "12.5 kg"/"1.29 kg/m"^3#
#color(white)("V"_"air") = "9.69 m"^3#
—————
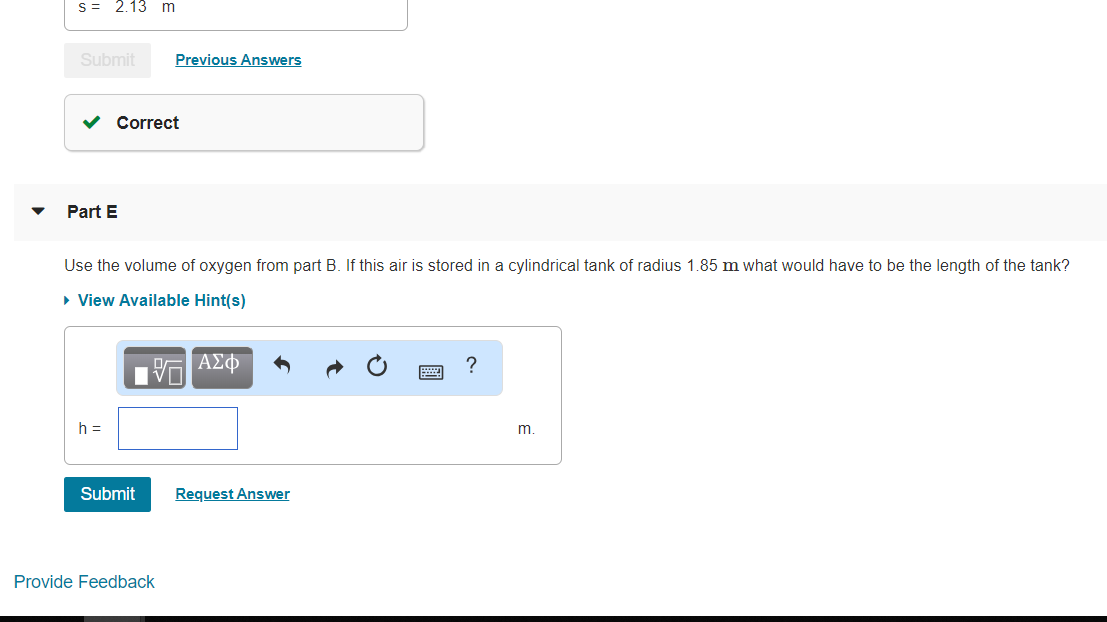
#"E."#
"Use Volume of oxygen from part B"
There is no mention of volume of oxygen in part B
If #"12.5 kg"# air whose volume is #"9.69 m"^3# (calculated in part C) is stored in cylinderical tank then height (#h#) of the cylinder is
Volume of cylinder#=# Volume of air#= pir^2h#
#h = "V"_"air"/(πr^2)#
#color(white)(h) = "9.69 m"^3/(3.14 × ("1.85 m")^2)#
#color(white)(h) = "0.902 m"#