Could someone please describe the shapes of electron density maps for s and p orbital? And why they're shaped the way they are?
1 Answer
See the illustrations below for the orbital shapes, and a discussion of why these shapes are used.
Explanation:
The
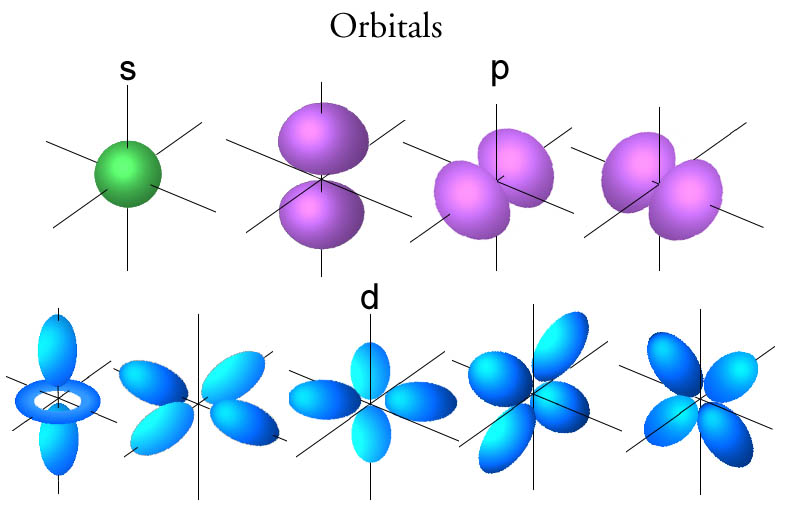
The
The reason these shapes arise is purely a mathematical calculation. When one solves the Schrodinger equation of the hydrogen atom, the solutions (called wavefunctions,
The orbitals represent the probability distribution found by multiplying each wavefunction by its complex conjugate (

