Could someone please explain to me what does it mean when an atom gets 'excited' and how they reach that state?
1 Answer
Oct 27, 2017
Atoms by default are usually in their ground state as their "preferred" state.
An atomic excited state is when an atom receives energy from some source that is then used to promote one or more electrons to higher energy levels.
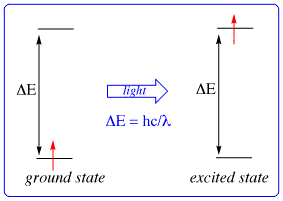
Excited states are naturally unstable.
They eventually die down and in essence, the electron(s) relax down to the lowest energy level, returning the atom back to its ground state.

