Describe the bonds and forces that stabilise the tertiary globular structure of proteins.?
1 Answer
Tertiary structure of a protein is a three-dimensional structure of a polypeptide and such structure has interactions between the R groups of the amino acids that make up the protein.

Explanation:
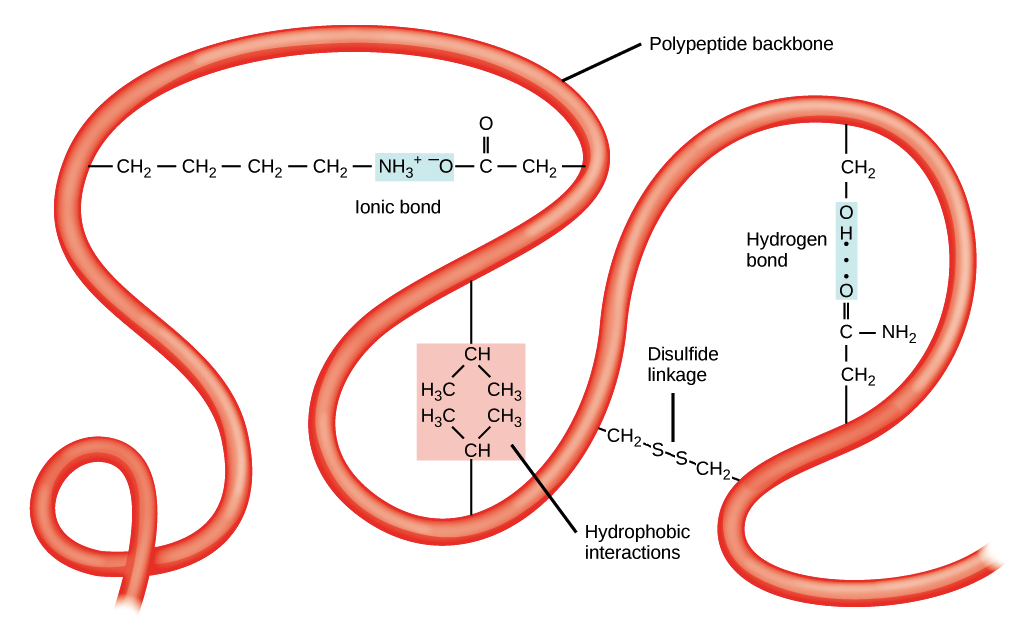
Interactions in the tertiary structure include:
-
Intramolecular forces
Amino acids, monomers of proteins, form peptide bonds [red color]
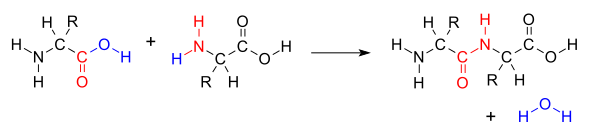
Picture 1: An amino group(NH2 in the second amino acid is covalently attached to the carboxyl group(COOH) of the first amino acid.
The result is a peptide bond (-CO-NH-)and a molecule of water. -
Intermolecular forces
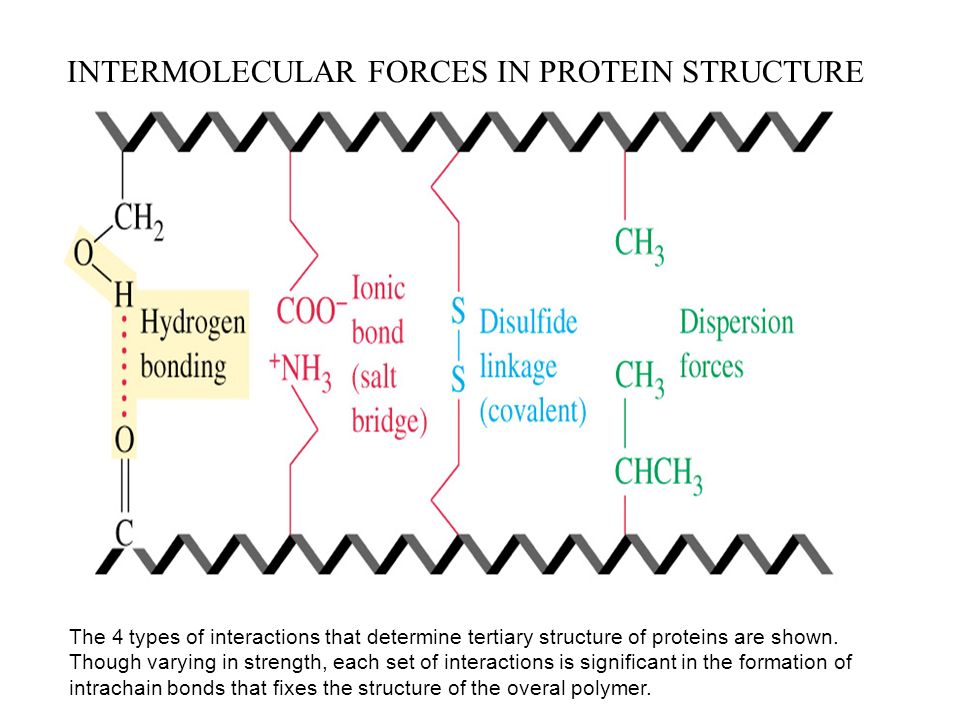
Hydrogen bonding, ionic bonding, dipole-dipole interactions, London dispersion forces and disulfide bonds
R groups with the same charges repel one another and those with opposite charges form an ionic bond.
Polar R groups can form hydrogen bonds and other dipole-dipole interactions.
Nonpolar, hydrophobic R groups cluster together on the inside of the protein
A globular protein has hydrophobic amino acids in the core and on the surface are hydophilic amino acids that are exposed to the water. Such arrangement may stabilise interactions in the tertiary structure.
Dispersion forces are present when two non-polar molecules [R gropus in amino acids] interact together.
The disulfide bond is a special type of covalen bond found in the tertiary structure.
Those covalent linkages between side chains of cysteines [an amino acid], are stronger than the other types of bonds in the tertiary structure. They keep parts of polypeptide attached and maintain its stability.

