Describe the composition of human haemoglobin, and explain how haemoglobin supports oxygen transport in the blood, using a diagram of the haemoglobin dissociation curve. 5 marks?
1 Answer
See Below - this will need a plot.!
Explanation:
Hemoglobin is the main oxygen transporting molecule in blood.
Hemoglobin is made from 2 alpha type subunits and 2 beta type subunits. It is the classic example of protein quaternary structure. Each subunit is a lot like an individual myoglobin unit, and each subunit contains a prosthetic heme group. Coordinated in this heme group is the Iron(II) ion that allows hemoglobin to bind oxygen.
Hemoglobin has the ability to bind 4 oxygen molecules. The coordinated iron(II) ion in the hemes can bind 1 oxygen each. However, they do not bind at the same time, but rather one at a time. This process of one at a time binding affinity leads to something called a sigmoidal binding curve. The first oxygen goes on poorly, but the next one is easier to go on, and so on...until the last one goes on easily. This shift is due to allosteric changes in the quaternary structure of the protein, and this phenomenon is what leads to hemoglobins ability to transport oxygen.
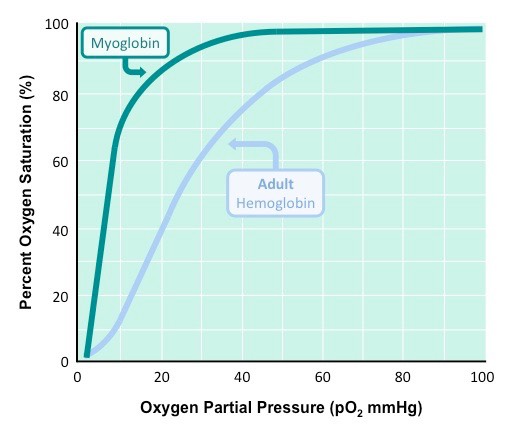
At high oxygen pressure, say in the lungs, both hemoglobin and myoglobin would be sauturated with oxygen. However, as the pressure of oxygen decreases (in the tissue at say 10 pO2, myoglobin still has almost all of its oxygen attached, but in the hemoglobin, only 10% is still attached.
So in high oxygen partial pressure environments (lungs), both molecules get bound with oxygen. In low oxygen environments (tissue...where oxygen is needed!), myoglobin holds onto is oxygen, but hemoglobin dumps it off (into the tissue where it is needed). This is how hemoglobin delivers oxygen to tissue - due mostly to its sigmoidal, or allosteric, oxygen binding curve....what is sometimes called cooperative binding.
