Details below. Acidity order "?"
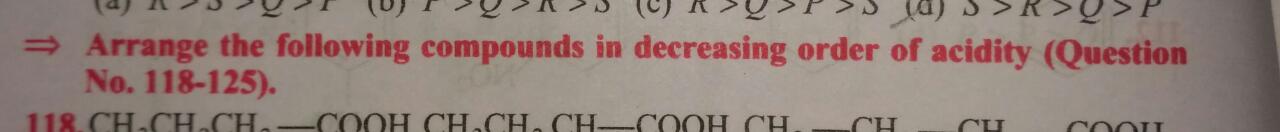
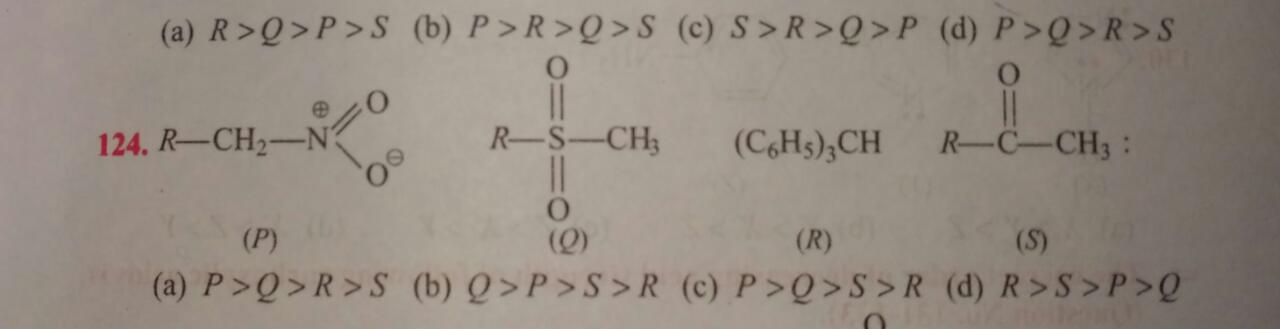
1 Answer
Your first question is cut off. I won't answer that because I don't know all of the information.
Regarding your second question: Consider

What happens when we deprotonate the carbon adjacent to the nitro functional group? The negative charge is delocalized via resonance.
Consider

When we deprotonate the methyl group, the negative charge can be delocalized to two oxygen atoms via resonance.
Consider

When we deprotonate the central carbon, the negative charge is delocalized among the three phenyl substituents via resonance.
Consider

When we deprotonate the carbon allylic to the carbonyl oxygen, its negative charge would delocalize to the oxygen via resonance.
Recall: more electronegative atoms will make for a more stable negative charge. In assessing acidity, we want to determine which of these potential acids has the most stable base.
From the preceding reasoning I would determine,
I don't really agree that the sulfone proton is more acidic than the carbonyl proton, but they're pretty close so I could see why one would argue the other way.

