Determine the number of electron groups around the central atom for each of the following molecules?
Hi! So like my question says, i need to determine the number of electron groups around the central atom for each of the following molecules:
NF3
OF2
H2S
I have tried entering the following and have gotten them wrong.
NF3 = 3
OF2 = 2
H2S = 2
Any explanation you could give would be greatly appreciated, thank you!
Hi! So like my question says, i need to determine the number of electron groups around the central atom for each of the following molecules:
NF3
OF2
H2S
I have tried entering the following and have gotten them wrong.
NF3 = 3
OF2 = 2
H2S = 2
Any explanation you could give would be greatly appreciated, thank you!
1 Answer
Electron groups are lone pairs and/or bonds (since we explain bonds as a pair of shared electrons).
Explanation:
For
If you look at the Nitrogen, it has 4 different electron groups around it (3 from the bonds, 1 from lone pair). You can re-draw the bonds as pairs of Electrons - this might help show how there are 4 groups.

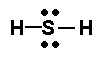
All of these have electron geometry of "Tetrahedral".
Molecular Geometry will be: Trigonal Pyramidal, Bent, Bent.

